Abstract
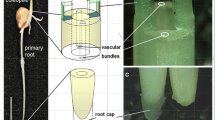
The starch-statolith theory of gravity reception has been tested with a mutant of Arabidopsis thaliana (L.) Heynh. which, lacking plastid phosphoglucomutase (EC 2.7.5.1) activity, does not synthesize starch. The hypocotyls and seedling roots of the mutant were examined by light and electron microscopy to confirm that they did not contain starch. In upright wild-type (WT) seedlings, starch-filled plastids in the starch sheath of the hypocotyl and in three of the five columellar layers of the root cap were piled on the cell floors, and sedimented to the ceilings when the plants were inverted. However, starchless plastids of the mutant were not significantly sedimented in these cells in either upright or inverted seedlings. Gravitropism of light-grown seedling roots was vigorous: e.g., 10o curvature developed in mutants rotated on a clinostat following a 5 min induction at 1 · g, compared with 14o in the WT. Curvatures induced during intervals from 2.5 to 30 min were 70% as great in the mutant as the WT. Thus under these conditions the presence of starch and the sedimentation of plastids are unnecessary for reception of gravity by Arabidopsis roots. Gravitropism by hypocotyls of light-grown seedlings was less vigorous than that by roots, but the mutant hypocotyls exhibited an average of 70–80% as much curvature as the WT. Roots and hypocotyls of etiolated seedlings and flower stalks of mature plants were also gravitropic, although in these cases the mutant was generally less closely comparable to the WT. Thus, starch is also unnecessary for gravity reception in these tissues.
Similar content being viewed by others
Abbreviations
- PAR:
-
photosynthetically active radiation
- PAS:
-
periodic acid-Schiff's reagent
- PGM:
-
phosphoglucomutase
- WT:
-
wild-type
References
Audus, L.J. (1962) The mechanism of the perception of gravity by plants. Symp. Soc. Exp. Biol. 16, 197–226
Audus, L.J. (1975) Geotropism in roots. In: Development and function of roots, pp. 327–363, Torrey, J.G., Clarkson, D.T., eds. Academic Press, London New York San Francisco
Barlow, P.B. (1974) Recovery of geotropism after removal of the root cap. J. Exp. Bot. 25, 1137–1146
Caspar, T., Huber, S.C., Somerville, C.R. (1985a) Alterations in growth, photosynthesis, and respiration in a starchless mutant of Arabidopsis thaliana (L.) deficient in chloroplast phosphoglucomutase activity. Plant Physiol. 79, 11–17
Caspar, T., Somerville, C.R., Pickard, B.G. (1985b) Geotropic roots and shoots of a starch-free mutant of Arabidopsis. (Abstr.) Plant Physiol. 77, Suppl., 105
Clifford, P.E. (1979) Amyloplast movement and the geotropic response. Z. Pflanzenphysiol. 91, 69–74
Clifford, P.E., Barclay, G.F. (1980) The sedimentation of amyloplasts in living statocytes of the dandelion flower stalk. Plant Cell Environ. 3, 381–386
Dolk, H.E. (1936) Geotropism and the growth substance. Rec. Trav. Bot. Néerl. 33, 509–585
Feder, N., O'Brien, T.P. (1968) Plant microtechnique: some principles and new methods. Am. J. Bot. 55, 123–142
Feldman, L.J. (1985) Root gravitropism. Physiol. Plant. 65, 341–344
Filner, B., Hertel, R., Steele, C., Fan, V. (1970) Some aspects of geotropism in coleoptiles. Planta 94, 333–354
Grenville, D.J., Peterson, R.L. (1981) Structure of aerial and subterranean roots of Selaginella kraussiana A. Br. Bot. Gaz. 142, 73–81
Haughn, G.W., Somerville, C.R. (1986) Sulfonylurea-resistant mutants of Arabidopsis thaliana. Mol. Gen. Genet. 204, 430–434
Hertel, R., Dela Fuente, R.K., Leopold, A.C. (1969) Geotropism and the lateral transport of auxin in the corn mutant amylomaize. Planta 88, 204–214
Hillman, S.K., Wilkins, M.B. (1982) Gravity perception in decapped roots of Zea mays. Planta 155, 267–271
Iversen, T.-H. (1969) Elimination of geotropic responsiveness in roots of cress (Lepidium sativum) by removal of statolith starch. Physiol. Plant. 22, 1251–1262
Iversen, T.-H., Larsen, P. (1973) Movement of amyloplasts in the statocytes of geotropically stimulated roots. The preinversion effect. Physiol. Plant. 28, 172–181
Jackson, M.B., Barlow, P.W. (1981) Root geotropism and the role of growth regulators from the cap: a re-examination. Plant Cell Environ. 4, 107–123
Johnsson, A., Pickard, B.G. (1979) The threshold stimulus for geotropism. Plant. Physiol. 45, 315–319
Juniper, B.E. (1976) Geotropism. Annu. Rev. Plant Physiol. 27, 385–406
Kiss, J.Z., Hertel, R., Sack, F.D. (1989) Amyloplasts are necessary for full gravitropic sensitivity in roots of Arabidopsis thaliana. Planta 177, 198–206
Klomparens, K.L., Flegler, S.L., Hooper, G.R. (1986) Procedures for transmission and scanning electron microscopy for biological and medical science. A laboratory manual, 2nd edn. Ladd Research Industries, Burlington, Vt., USA
Langhaar, H.L. (1951) Dimensional analysis and theory of models. John Wiley and Sons, New York
Larsen, P. (1957) The development of geotropic and spontaneous curvatures in roots. Physiol. Plant. 10, 127–163
Larsen, P. (1971) The susception of gravity by higher plants. In: Gravity and the organism, pp. 73–87, Gordon, S.A., Cohen, M.J., eds. University of Chicago Press, Chicago
Lin, T.P., Caspar, T., Somerville, C.R., Preiss, J. (1988) Isolation and characterization of a starchless mutant of Arabidopsis thaliana (L.) Heynh. lacking ADPglucose pyrophosphorylase activity. Physiol. Plant. 86, 1131–1135
Mackinney, G. (1941) Absorption of light by chlorophyll solutions. J. Biol. Chem. 140, 315–322
Miles, D. (1981) The relationship of geotropic response and statolith in a nuclear mutant of maize. (Abstr.) Physiol. Plant. 67, Suppl., 100
Mirza, J.L., Olsen, G.M., Iversen, T.-H., Maher, E.P. (1984) The growth and gravitropic responses of wild-type and auxin-resistant mutants of Arabidopsis thaliana. Physiol. Plant. 60, 516–522
Moore, R. (1987) Root gravitropism in a cultivar of Zea mays whose columella cells contain starch-deficient amyloplasts. Ann. Bot. 59, 661–666
Moore, R., Evans, M.L. (1986) How roots perceive and respond to gravity. Am. J. Bot. 73, 574–587
Olsen, G.M., Mirza, J.I., Maher, P., Iversen, T.-H. (1984) Ultrastructure and movements of cell organelles in the root cap of agravitropic mutants and normal seedlings of Arabidopsis thaliana. Physiol. Plant. 60, 523–531
Perbal, G., Rivière, S. (1976) Relation entre réaction géotropique et évolution du statenchyme dans la racine d'Asperge. Physiol. Plant. 38, 39–47
Pickard, B.G. (1971) The susception of gravity by higher plants: analysis of geotonic data for theories of georeception. In: Gravity and the organism, pp. 89–96, Gordon, S.A., Cohen, M.J., eds. University of Chicago Press, Chicago
Pickard, B.G. (1973) Geotropic response patterns of the Avena coleoptile. I. Dependence on angle and duration of stimulation. Can. J. Bot. 51, 1003–1021
Pickard, B.G., Thimann, K.V. (1966) Geotropic response of wheat coleoptiles in absence of amyloplast starch. J. Gen. Physiol. 49, 1065–1086
Pierce, J.W., McCurry, S.D., Mulligan, R.M., Tolbert, N.E. (1982) Activation and assay of ribulose-1,5-bisphosphate carboxylase/oxygenase. Methods Enzymol. 89, 47–55
Quail, P.H. (1979) Plant cell fractionation. Annu. Rev. Plant. Physiol. 30, 425–484
Reynolds, E.S. (1963) The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J. Cell. Biol. 17, 208–212
Roberts, J.A. (1984) Tropic responses of hypocotyls from normal tomato plants and the agravitropic mutant Lazy-1. Plant Cell Environ. 7, 515–520
Sack, F.D., Suyemoto, M.M., Leopold, A.C. (1984) Kinetics of amyloplast sedimentation in gravistimulated maize coleoptiles. Planta 161, 459–464
Shen-Miller, J., Hinchman, R.R. (1974) Gravity sensing in plants: a critique of the statolith theory. BioScience 24, 643–651
Somerville, C.R., Somerville, S.C., Ogren, W.L. (1981) Isolation of photosynthetically active protoplasts and chloroplasts from A. thaliana. Plant Sci. Lett. 21, 89–96
Stitt, M., Bulpin, P.V., ap Rees, T. (1978) Pathway of starch breakdown in photosynthetic tissues of Pisum sativum. Biochim. Biophys. Acta. 544, 200–214
Volkmann, D., Sievers, A. (1979) Graviperception in multicellular organisms. In: Encyclopedia of plant physiology, N.S., vol. 7: Physiology of movements, pp. 573–600, Haupt, W., Feinleib, M.E., eds. Springer, Berlin Heidelberg New York
Wendt, M., Sievers, A. (1986) Restitution of polarity in statocytes from centrifuged roots. Plant Cell Environ. 9, 17–23
Weibel, W.R., Bolender, R.P. (1972) Stereological techniques for electron microscopic morphometry. In: Principles and techniques of electron microscopy: biological applications, vol. 3, pp. 239–296, Hayat, M.A., ed. van Nostrand Reinhold Co., New York
Westing, A.H. (1971) A case against statoliths. In: Gravity and the organism, pp. 97–104, Gordon, S.A., Cohen, M.J., eds., University of Chicago Press, Chicago
Wilkins, M.B. (1984) Gravitropism. In: Advanced plant physiology, pp. 163–185, Wilkins, M.B., ed., Pitman, London
Wright, M. (1986) The acquisition of gravisensitivity during the development of nodes of Avena fatua. J. Plant Growth Regul. 5, 37–47
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Caspar, T., Pickard, B.G. Gravitropism in a starchless mutant of Arabidopsis . Planta 177, 185–197 (1989). https://doi.org/10.1007/BF00392807
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00392807