Abstract
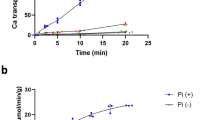
The Archaebacterium Haloferax volcanii concentrates K+ up to 3.6 M. This creates a very large K+ ion gradient of between 500- to 1,000-fold across the cell membrane. H. volcanii cells can be partially depleted of their internal K+ but the residual K+ concentration cannot be lowered below 1.5 M. In these conditions, the cells retain the ability to take up potassium from the medium and to restore a high internal K+ concentration (3 to 3.2 M) via an energy dependent, active transport mechanism with a K m of between 1 to 2 mM. The driving force for K+ transport has been explored. Internal K+ concentration is not in equilibrium with ΔΨm suggesting that K+ transport cannot be accounted for by a passive uniport process. A requirement for ATP has been found. Indeed, the depletion of the ATP pool by arsenate or the inhibition of ATP synthesis by N,N′-dicyclohexylcarbodiimide inhibits by 100% K+ transport even though membrane potential ΔΨm is maintained under these conditions. By contrast, the necessity of a ΔΨm for K+ accumulation has not yet been clearly demonstrated. K+ transport in H. volcanii can be compared with K+ transport via the Trk system in Escherichia coli.
Similar content being viewed by others
Abbreviations
- CCCP:
-
Carbonylcyanide m-chlorophenyl-hydrazone
- DCCD:
-
N,N′-dicyclohexylcarbodiimide
- MES:
-
2-[N-morpholino] ethane sulfonic acid
- MOPS:
-
3-[N-morpholino] propane sulfonic acid
- TRIS:
-
Tris (hydroxymethyl) aminomethane
- TPP:
-
tetraphenyl phosphonium
References
Bakker ER, Rottenberg H, Caplan SR (1976) An estimation of the light-induced electrochemical potential difference of protons across the membrane of Halobacterium halobium. Biochim Biophys Acta 440:557–572
Epstein W, Whitelaw V, Hesse J (1978) A K+ transport ATPase in Escherichia coli. J Biol Chem 253:6666–6668
Garty H, Caplan R (1977) Light-dependent rubidium transport in intact Halobacterium halobium cells. Biochim Biophys Acta 459:532–545
Ginzburg M, Sachs L, Ginzburg B-Z (1971) Ion metabolism in Halobacterium halobium. J Membr Biol 5:78–101
Juez G (1988) Taxonomy of extremely halophilic archaebacteria. In: Rodriguez-Valera F (ed) Halophilic bacteria, vol II. CRC, Boca Raton, pp 3–24
Klein WL, Boyer PD (1972) Energization of active transport by Escherichia coli. J Biol Chem 247:7257–7265
Konishi T, Murakami N (1984) Detection of two DCCD-binding components in the envelope membrane of Halobacterium halobium. FEBS Lett 169:283–286
Konishi T, Murakami N (1988) ΔΨ-dependent gating of Na+/H+ exchange in Halobacterium halobium: a ΔμH+-driven Na+ pump. FEBS Lett 226:270–274
Lanyi JK, Hilliker K (1976) Passive potassium ion permeability of Halobacterium halobium envelope membranes. Biochim Biophys Acta 448:181–184
Lanyi JK, Silverman MP (1972) The state of binding of intracellular K+ in Halobacterium cutirubrum. Can J Microbiol 18:993–995
Lanyi JK, Helgerson S, Silverman M (1979) Relationship between proton motive force and potassium ion transport in Halobacterium halobium vesicles envelope. Arch Biochem Biophys 193:329–339
Meury J (1976) Transport du potassium chez Escherichia coli. Thesis. Univ. Paris
Meury J, Lebail S, Kepes A (1980) Opening of potassium channels in Escherichia coli membranes by thiol reagents and recovery of potassium tightness. Eur J Biochem 113:33–38
Meury J, Kepes A (1981) The regulation of potassium fluxes for the adjustment and maintenance of potassium levels in Escherichia coli. Eur J Biochem 19:165–170
Mevarech M, Werczberger R (1985) Genetic transfer in Halobacterium volcanii. J Bacteriol 162:461–462
Murakami N, Konishi T (1985) DCCD-sensitive, Na+-dependent H+-influx process coupled to membrane potential formation in membrane vesicles of Halobacterium halobium. J Biochem (Tokyo) 98:897–907
Rhoads DB, Epstein W (1977) Energy coupling to net K+ transport in Escherichia coli K-12. J Biol Chem 253:1394–1401
Richarme G (1985) Possible involvement of lipoic acid in binding protein-dependent transport systems in Escherichia coli. J Bacteriol 162:286–293
Steward LMD, Bakker EP, Booth IR (1985) Energy coupling to K+ uptake via the Trk system in Escherichia coli: the role of ATP. J Gen Microbiol 131:77–85
Tokuda H, Nakamura T, Unemoto T (1981) Potassium ion is required for the generation of pH-dependent membrane potential and ΔpH by the marine bacterium Vibrio alginolyticus. Biochemistry 20:4198–4203
Wagner G, Hartmann R, Oesterhelt D (1978) Potassium uniport and ATP synthesis in Halobacterium halobium. Eur J Biochem 89:160–179
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Meury, J., Kohiyama, M. ATP is required for K+ active transport in the archaebacterium Haloferax volcanii . Arch. Microbiol. 151, 530–536 (1989). https://doi.org/10.1007/BF00454870
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00454870