Summary
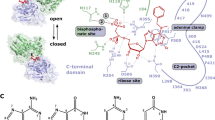
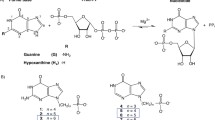
Purine nucleoside phosphorylase catalyzes the reversible phosphorolysis of purine ribonucleosides and 2′-deoxyribonucleosides to the free base and ribose-1-phosphate or 2′-deoxyribose-1-phosphate. PNP isolated from humans is specific for guanosine, inosine and certain analogs, although PNPs from other organisms show varying levels of specificity. Interest in PNP arises from its critical role in purine nucleoside metabolism and in T-cell function, which indicates that inhibitors of this enzyme might be useful in the treatment of T-cell proliferative diseases such as T-cell leukemias and lymphomas, in the suppression of host versus graft response in organ transplant patients, and in the treatment of T-cell-mediated autoimmune diseases. Most potent inhibitors of this enzyme are derivatives of guanine or 8-aminoguanine, including 9-arylmethyl derivatives. Of these, 9-benzylguanines substituted at position 2 or 3 of the phenyl ring by a side chain terminating in a phosphonate moiety are the most potent, with Ki in the nanomolar range. However, these compounds have limited potential as drugs because of their very low cell permeability. As a result, inhibitors of this type that have been tested are effective in whole cells only at concentrations of 100 µM or higher. On the other hand, the three-dimensional structure of human PNP, determined by X-ray crystallography, has been used in designing novel inhibitors of this key enzyme, resulting in several families of membrane-permeable inhibitors with IC50 values in the 6—30 nM range. The inhibition is competitive with respect to both inosine and phosphate. 9-(3-Pyridylmethyl)-9-deazaguanine (BCX-34) was chosen from these inhibitors for further study. Its Ki for the inhibition of human erythrocytic PNP is 31 nM, and its IC50 for inhibition of the proliferation of T cells (CCRF-CEM) is 1.4 µM in the presence of 5.6 µM of 2′-deoxyguanosine, which alone has no effect on cells. It did not inhibit the proliferation of B cells (MGL-8) up to 30 µM. Phase I/II clinical studies of a dermal formulation of BCX-34 have shown this drug to be efficacious and safe in the treatment of cutaneous T-cell lymphoma and psoriasis.
Similar content being viewed by others
References
Parks Jr., R.E., Stoeckler, J.D., Cambor, D., Savarese, T.M., Crabtree, G.W. and Chu, S.-H., In Sartorelli, A., Lazo, J.S. and Bertino, J.R. (Eds.) Molecular Actions and Targets for Cancer Chemotherapeutic Agents, Academic Press, New York, NY, 1981, pp. 229–252.
Stoeckler, J.D., In Glazer, R.I. (Ed.) Developments in Cancer Chemotherapy, CRC Press, Boca Raton, FL, 1984, pp. 35–60.
Ealick, S.E., Rule, S.A., Carter, D.C., Greenhough, T.J., Babu, Y.S., Cook, W.J., Habash, J., Helliwell, J.R., Stoeckler, J.D., Parks Jr., R.E., Chen, S.-F. and Bugg, C.E., J. Biol. Chem., 265 (1990) 1812.
Ealick, S.E., Babu, Y.S., Bugg, C.E., Erion, M.D., Guida, W.C., Montgomery, J.A. and Secrist III, J.A., Proc. Natl. Acad. Sci. USA, 88 (1991) 11540.
Zimmerman, T.P., Gersten, N.B., Ross, A.F. and Miech, R.P., Can. J. Biochem., 49 (1971) 1050.
Cory, J.G. and Cory, A.H. (Eds.) Inhibitors of Ribonucleoside Diphosphate Reductase Activity, Pergamon Press, New York, NY, 1989.
Markert, M.L., Immunodefic. Rev., 3 (1991) 45.
Otterness, I.G. and Bliven, M.L., In Rainsford, K.D. and Velo, G.P. (Eds.) New Developments in Antirheumatic Therapy, Kluwer Academic, Norwell, MA, 1989, pp. 277–304.
Montgomery, J.A., Med. Res. Rev., 13 (1993) 209.
Williams, S.R., Goddard, J.M. and Martin Jr., D.W., Nucleic Acids Res., 12 (1984) 5779.
Montgomery, J.A., Niwas, S., Rose, J.D., Secrist III, J.A., Babu, Y.S., Bugg, C.E., Erion, M.D., Guida, W.C. and Ealick, S.E., J. Med. Chem., 36 (1993) 55.
Secrist III, J.A., Niwas, S., Rose, J.D., Babu, Y.S., Bugg, C.E., Erion, M.D., Guida, W.C., Ealick, S.E. and Montgomery, J.A., J. Med. Chem., 36 (1993) 1847.
Erion, M.D., Niwas, S., Rose, J.D., Ananthan, S., Allen, M., Secrist III, J.A., Babu, Y.S., Bugg, C.E., Guida, W.C., Ealick, S.E. and Montgomery, J.A., J. Med. Chem., 36 (1993) 3771.
Stoeckler, J.D., Ryden, J.B., Parks Jr., R.E., Chu, M.-Y., Lim, M.-I., Ren, W.-Y. and Klein, R.S., Cancer Res., 46 (1986) 1774.
Kazmers, I.S., Mitchell, B.S., Daddona, P.E., Wotring, L.L., Townsend, L.B. and Kelley, W.N., Science, 214 (1981) 1137.
Osborne, W.R.A. and Barton, R.W., Immunology, 59 (1986) 63.
Slichter, S.J., Deeg, H.J. and Osborne, R.A., Br. J. Haematol., 75 (1990) 591.
Benear, J.B., Frederick, D., Townsend, L. and Epstein, R.B., Transplantation, 41 (1986) 274.
Shewach, D.S., Chern, J.-W., Pillote, K.E., Townsend, L.B. and Daddona, P.E., Cancer Res., 46 (1986) 519.
Sircar, J.C. and Gilbertsen, R.B., Drugs Future, 13 (1988) 653.
Burley, S.K. and Petsko, G.A., Science, 229 (1985) 23.
Woo, P.W.K., Kostlan, C.R., Sircar, J.C., Dong, M.K. and Gilbertsen, R.B., J. Med. Chem., 35 (1992) 1451.
Yen, C.Y., Chern, J.W. and Wang, H.H., Chin. Pharm. J., 42 (1990) 249.
Gilbertsen, R.B., Scott, M.E., Dong, M.K., Kossarek, L.M., Bennett, M.K., Schrier, D.J. and Sircar, J.C., Agents Actions, 21 (1987) 272.
Gilbertsen, R.B., Dong, M.K. and Kossarek, L.M., Agents Actions, 22 (1987) 379.
Gilbertsen, R.B., Josyula, U., Sircar, J.C., Dong, M.K., Wu, W., Wilburn, D.J. and Conroy, M.C., Biochem. Pharmacol., 44 (1992) 996.
Stein, J.M., Stoeckler, J.D., Li, S.-Y., Tolman, R.L., MacCoss, M., Chen, A., Karkas, J.D., Ashton, W.T. and Parks Jr., R.E., Biochem. Pharmacol., 36 (1987) 1237.
Bzowska, A., Kulikowska, E., Shugar, D., Bing-Yi, C., Lindborg, B. and Johansson, N.G., Biochem. Pharmacol., 41 (1991) 1791.
Tuttle, J.V. and Krenitsky, T.A., J. Biol. Chem., 259 (1984) 4065.
Krenitsky, T.A., Tuttle, J.V., Miller, W.H., Moorman, A.R., Orr, G.F. and Beauchamp, L., J. Biol. Chem., 265 (1990) 3066.
Nakamura, C.E., Chu, S.-H., Stoeckler, J.D. and Parks Jr., R.E., Biochem. Pharmacol., 35 (1986) 133.
Nakamura, C.E., Chu, S.-H., Stoeckler, J.D. and Parks Jr., R.E., Nucleosides Nucleotides, 8 (1989) 1039.
Babu, Y.S. and Montgomery, J.A., unpublished data.
Halazy, S., Ehrhard, A. and Danzin, C., J. Am. Chem. Soc., 113 (1991) 315.
Mamont, P.S., Weibel, M., Danzin, C. and Halazy, S., Int. J. Pur. Pyr. Res., 2 (1991) 62.
Sediva, K., Ananiev, A. V., Votruba, I., Holy, A. and Rosenberg, I., Int. J. Pur. Pyr. Res., 2 (1991) 35.
Jordan, F. and Wu, A., Arch. Biochem. Biophys., 190 (1978) 699.
Salamone, S.J. and Jordan, F., Biochemistry, 21 (1982) 6383.
Dempcy, R.O. and Skibo, E.B., Biochemistry, 30 (1991) 8480.
Willis, R.C., Robins, R.K. and Seegmiller, J.E., Mol. Pharmacol., 18 (1980) 287.
Erickson, J.W. and Fesik, S.W., Annu. Rep. Med. Chem., 27 (1992) 271.
Halazy, S., Eggenspiller, A., Ehard, A. and Dancin, C., Bioorg. Med. Chem. Lett., 2 (1992) 407.
Kelly, J.L., Linn, J.A., McLean, E.W. and Tuttle, J.V., Abstract Med:104 of the 205th ACS National Meeting, Denver, CO, March 28–April 2, 1993.
Montgomery, J.A., Snyder Jr., H.W., Walsh, D.A. and Walsh, G.M., Drugs Future, 18 (1993) 887.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Montgomery, J.A., Secrist, J.A. PNP inhibitors. Perspectives in Drug Discovery and Design 2, 205–220 (1994). https://doi.org/10.1007/BF02171744
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02171744