Abstract
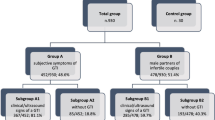
We have shown that patients with prostato-vesiculo-epididymitis (PVE) have the worst sperm output compared to patients with prostato-vesiculitis or prostatitis alone. The present study was undertaken to closely examine whether unilateral or bilateral PVE had a different impact on sperm parameters, seminal fructose levels and reactive oxygen species (ROS) overproduction. To accomplish this, 78 patients with persistent post-infectious inflammatory PVE, clearly identified by scrotal and transrectal ultrasonography, and 30 patients with asymptomatic post-infectious inflammatory prostatitis (control group) underwent semen analysis (including seminal leukocyte concentration and number of spermiophagies), seminal fructose measurement and sperm ROS production from 45 and 90% Percoll fractions. Fifty patients turned out to have PVE bilaterally, whereas the remaining 28 had unilateral PVE. Patients with bilateral PVE had sperm concentration and total sperm number significantly lower than those found in patients with unilateral PVE. The other sperm parameters, the physicochemical properties (hyperviscosity, the presence of nonspecific agglutination, delayed liquefaction), seminal fructose levels and ROS production in both 45 and 90% Percoll fractions turned out similar between the two groups. Patients with bilateral or unilateral PVE had sperm parameters, seminal fructose levels and ROS production significantly worst than those found in patients with prostatitis alone. In conclusion, although patients with bilateral PVE had a decreased number of spermatozoa, the other sperm parameters and seminal fructose levels did not reflect the extension of PVE. Therefore, the diagnosis of unilateral or bilateral involvement of this complicated form of male accessory gland infection relies on scrotal and transrectal ultrasonography.
Similar content being viewed by others
References
World Health Organization. WHO manual for the standardized investigation and diagnosis of the infertile couple. Cambridge, New York: Cambridge University Press 1993.
Nickel JC. Classification and diagnosis of prostatitis: a gold standard? Andrologia 2003, 35: 160–7.
Weidner W, Krause W, Ludwig M. Relevance of male accessory gland infection for subsequent fertility with special focus on prostatitis. Hum Reprod Update 1999, 5: 421–32.
Vicari E. Seminal leukocyte concentration and related specific reactive oxygen species production in patients with male accessory gland infections. Hum Reprod 1999, 14: 2025–30.
Schill WB, Henkel R. Advancement in biochemical assays in andrology. Asian J Androl 1999, 1: 45–51.
World Health Organization. WHO laboratory manual for the examination of human semen and semen-cervical mucus interaction, 4th ed. Cambridge, UK: Cambridge University Press 1999.
Gonzales GF, Villena A. True corrected seminal fructose level: a better marker of the function of seminal vesicle in infertile men. Int J Androl 2001, 24: 255–60.
Vicari E. Effectiveness and limits of antimicrobial treatment on seminal leukocyte concentration and related reactive oxygen species production in patients with male accessory gland infection. Hum Reprod 2000, 15: 2536–44.
Cohen J, Edwards R, Fehilly C, et al. In vitro fertilization: a treatment for male infertility. Fertil Steril 1985, 43: 422–32.
Phadke AM. Neutral red supravital staining for cellular elements in the semen. Andrologia 1978, 10: 80–4.
Mann T, Lutwak-Mann C. Evaluation of the functional state of male accessory glands by the analysis of seminal plasma. Andrologia 1976, 8: 237–42.
Purvis K, Christiansen E. Infection in the male reproductive tract. Impact, diagnosis and treatment in relation to male infertility. Int J Androl 1993, 16: 1–13.
Kim ED, Lipschultz LI. Role of ultrasound in the assessment of male infertility. J Clin Ultrasound 1996, 24: 437–53.
Schipper RA, Trum JW, Messelink EJ, van der Veen F, Kurth KH. Transrectal ultrasonography in male subfertility patients: an intra- and interobserver study. Urol Res 2001, 29: 57–9.
Furuya R, Takahashi S, Furuya S, Kunishima Y, Takeyama K, Tsukamoto T. Is seminal vesiculitis a discrete disease entity? Clinical and microbiological study of seminal vesiculitis in patients with acute epididymitis. J Urol 2004, 171: 1550–3.
Vicari E, La Vignera S, Calogero AE. Antioxidant treatment with carnitines is effective in infertile patients with prostato-vesciculo-epididymitis and elevated seminal leukocyte concentration after treatment with non-steroidal anti-inflammatory compounds. Fertil Steril 2002, 78: 1203–8.
Comhaire FH, Mahmoud AM, Depuydt CE, Zalata AA, Christophe AB. Mechanisms and effects of male genital tract infection on sperm quality and fertilized potential: the andrologist’s viewpoint. Hum Reprod Update 1999, 5: 393–8.
Pasqualotto FF, Sharma RK, Potts JM, Nelson DR, Thomas AJ, Agarwal A. Seminal oxidative stress in patients with chronic prostatitis. Urology 2000, 55: 881–5.
Gonzales G F. Function of seminal vesicles and their role on male fertility. Asian J Androl 2001, 3: 251–8.
Wang A, Fanning L, Anderson DJ, Loughlin KR. Generation of reactive oxygen species by leukocytes and sperm following exposure to urogenital tract infection. Arch Androl 1997, 39: 11–7.
Vicari E, Calogero AE. Effect of treatment with carnitines in patients with prostato-vesiculo-epididymitis. Hum Reprod 2001, 16: 2338–42.
Ochsendorf FR. Infections in the male genital tract and reactive oxygen species. Hum Reprod Update 1999, 5: 399–420.
Gonzales G F, Kortebani G, Mazzolli AB. Leukocytospermia and function of the seminal vesicles on seminal quality. Fertil Steril 1992, 57: 1058–65.
Potts RJ, Jefferies TM, Notarianni LJ. Antioxidant capacity of the epididymis. Hum Reprod 1999, 14: 2513–6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vicari, E., La Vignera, S., Castiglione, R. et al. Sperm parameter abnormalities, low seminal fructose and reactive oxygen species overproduction do not discriminate patients with unilateral or bilateral post-infectious inflammatory prostato-vesiculo-epididymitis. J Endocrinol Invest 29, 18–25 (2006). https://doi.org/10.1007/BF03349172
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03349172