Abstract
Objective
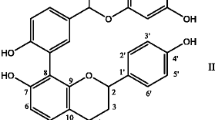
The aim of the present study was to investigate the cardioprotective effects of baicalein, main bioactive constituent from roots of Scutellaria baicalensis and Scutellaria lateriflora, on isoproterenol (ISO) induced acute myocardial infarction model in rats and to explore the underlying mechanisms.
Method
Rats were treated with baicalein (50 mg/kg and 100 mg/kg) orally for 14 days and on 13th and 14th day, myocardial injury was induced by ISO injection (100 mg/kg, subcutaneous) at an interval of 24 h.
Result
Our study showed that ISO administration resulted in significant elevations in the levels of cardiac injury biomarkers such as cardiac troponin I, creatine kinase-MB, AST and ALT. Concentrations of reactive nitrogen species and reactive oxygen species in the heart tissue increased significantly while antioxidant enzymes level declined. The levels of tissue pro-inflammatory cytokines tumor necrosis factor-α and interleukin-6 were significantly increased after ISO administration. Pretreatment with baicalein significantly reversed these alterations induced by ISO administration. Exploration of the underlying mechanisms of protective effect of baicalein pretreatment revealed that it repressed the expression of nuclear factor kappa B and restored the ISO induced elevation of pro-inflammatory cytokines, oxidative and nitrosative stress. We found that baicalein pretreatment enhanced the level of antioxidant defense enzymes like SOD, catalase and GSH. Furthermore, the present study also demonstrated cardioprotective effects of baicalein by the histopathological findings.
Conclusion
Taken together, our findings demonstrated that baicalein pretreatment might have a potential benefit in prevention and terminating ischemic heart diseases like myocardial infarction.






Similar content being viewed by others
Abbreviations
- ISO:
-
Isoproterenol
- MI:
-
Myocardial infarction
- NF-κB:
-
Nuclear factor kappa-light-chain enhancer of activated B cells
- TNF-α:
-
Tumor necrosis factor α
- IL-6:
-
Interleukin 6
- IL-10:
-
Interleukin 10
- SOD:
-
Superoxide dismutase
- MDA:
-
Malondialdehyde
- GSH:
-
Glutathione
References
Alla F, Zannad F, Filippatos G. Epidemiology of acute heart failure syndromes. Heart Fail Rev. 2007;12(2):91–5.
Aronow WS. Epidemiology, pathophysiology, prognosis, and treatment of systolic and diastolic heart failure. Cardiol Rev. 2006;14(3):108–24.
Patel V, Upaganlawar A, Zalawadia R, Balaraman R. Cardioprotective effect of melatonin against isoproterenol induced myocardial infarction in rats: a biochemical, electrocardiographic and histoarchitectural evaluation. Eur J Pharmacol. 2010;644(1–3):160–8.
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. J Am CollCardiol. 2012;60(16):1581–98.
Sawyer DB, Siwik DA, Xiao L, Pimentel DR, Singh K, Colucci WS. Role of oxidative stress in myocardial hypertrophy and failure. J Mol Cell Cardiol. 2002;34(4):379–88.
Bagatini MD, Martins CC, Battisti V, Gasparetto D, Da Rosa CS, Spanevello RM, et al. Oxidative stress versus antioxidant defenses in patients with acute myocardial infarction. Heart Vessels. 2011;26(1):55–63.
Wang S, Tian S, Yang F, Yang H, Yang X, Du G. Cardioprotective effect of salvianolic acid A on isoproterenol-induced myocardial infarction in rats. Eur J Pharmacol. 2009;615(1–3):125–32.
Rona G. Catecholamine cardiotoxicity. J Mol Cell Cardiol. 1985;17(4):291–306.
Rona G, Chappel CI, Balazs T, Gaudry R. An infarct-like myocardial lesion and other toxic manifestations produced by isoproterenol in the rat. AMA Arch Pathol. 1959;67(4):443–55.
Yates JC, Beamish RE, Dhalla NS. Ventricular dysfunction and necrosis produced by adrenochrome metabolite of epinephrine: relation to pathogenesis of catecholamine cardiomyopathy. Am Heart J. 1981;102(2):210–21.
Brooks WW, Conrad CH. Isoproterenol-induced myocardial injury and diastolic dysfunction in mice: structural and functional correlates. Comp Med. 2009;59(4):339–43.
Rajadurai M, Prince PSM. Preventive effect of naringin on lipid peroxides and antioxidants in isoproterenol-induced cardiotoxicity in Wistar rats: biochemical and histopathological evidences. Toxicology. 2006;228(2):259–68.
Prince PSM, Rajakumar S, Dhanasekar K. Protective effects of vanillic acid on electrocardiogram, lipid peroxidation, antioxidants, proinflammatory markers and histopathology in isoproterenol induced cardiotoxic rats. Eur J Pharmacol. 2011;668(1–2):233–40.
Zhou R, Xu Q, Zheng P, Yan L, Zheng J, Dai G. Cardioprotective effect of fluvastatin on isoproterenol-induced myocardial infarction in rat. Eur J Pharmacol. 2008;586(1–3):244–50.
Mu X, He G, Cheng Y, Li X, Xu B, Du G. Baicalein exerts neuroprotective effects in 6-hydroxydopamine-induced experimental parkinsonism in vivo and in vitro. Pharmacol Biochem Behav. 2009;92(4):642–8.
Wang CZ, Li XL, Wang QF, Mehendale SR, Yuan CS. Selective fraction of Scutellaria baicalensis and its chemopreventive effects on MCF-7 human breast cancer cells. Phytomedicine. 2010;17(1):63–8.
Shao ZH, VandenHoek TL, Qin Y, Becker LB, Schumacker PT, Li CQ, et al. Am J Physiol Heart Circ Physiol. 2002;282(3):H999–1006.
Woo AY, Cheng CH, Waye MM. Baicalein protects rat cardiomyocytes from hypoxia/reoxygenation damage via a prooxidant mechanism. Cardiovasc Res. 2005;65(1):244–53.
Gabrielska J, Oszmiański J, Zyłka R, Komorowska M. Antioxidant activity of flavones from Scutellaria baicalensisin lecithin liposomes. Z Naturforsch C. 1997;52(11–12):817–23.
De Carvalho RS, Duarte FS, de Lima TC. Involvement of GABAergic non-benzodiazepine sites in the anxiolytic-like and sedative effects of the flavonoid baicalein in mice. Behav Brain Res. 2011;221(1):75–82.
Xiong Z, Jiang B, Wu PF, Tian J, Shi LL, Gu J, et al. Antidepressant effects of a plant-derived flavonoid baicalein involving extracellular signal-regulated kinases cascade. Biol Pharm Bull. 2011;34(2):253–9.
Hsieh CJ, Hall K, Ha T, Li C, Krishnaswamy G, Chi DS. Baicalein inhibits IL-1beta- and TNF-alpha-induced inflammatory cytokine production from human mast cells via regulation of the NF-kappaB pathway. Clin Mol Allergy. 2007;26(5):5.
Deschamps JD, Kenyon VA, Holman TR. Baicalein is a potent in vitro inhibitor against both reticulocyte 15-human and platelet 12-human lipoxygenases. Bioorg Med Chem. 2006;14(12):4295–301.
Lin CC, Shieh DE. The anti-inflammatory activity of Scutellariarivularis extracts and its active components, baicalin, baicalein and wogonin. Am J Chin Med. 1996;24(1):31–6.
Panda VS, Naik SR. Cardioprotective activity of Ginkgo bilobaphytosomes in isoproterenol induced myocardial necrosis in rats: a biochemical and histoarchitectural evaluation. Exp Toxicol Pathol. 2008;60(4–5):397–404.
Budhani MK, Dahiya V, Kasala ER, Bodduluru LN, Sharma D, Lahkar M, Kumar V. Animal models of myocardial infarction: mainstay in clinical translation. Regul Toxicol Pharmacol. 2016;. doi:10.1016/j.yrtph.2016.03.005.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–8.
Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47(2):389–94.
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–7.
Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harb Protoc. 2008. doi:10.1101/pdb.prot4986.
Deng X-Y, Chen J-J, Li H-Y, Ma Z-Q, Ma S-P, Fu Q. Cardioprotective effects of timosaponin B II from Anemarrhenae asphodeloides Bge on isoproterenol-induced myocardial infarction in rats. Chem Biol Int. 2015;240:22–8.
Kasala ER, Bodduluru LN, Barua CC, Madana RM, Dahiya V, Budhani MK et al. Chemopreventive effect of chrysin, a dietary flavone against benzo (a) pyrene induced lung carcinogenesis in Swiss albino mice. Pharmacol Rep. doi:10.1016/j.pharep.2015.08.014.
Booth EA, Lucchesi BR. Estrogen-mediated protection in myocardial ischemia-reperfusion injury. Cardiovasc Toxicol. 2008;8(3):101–13.
Deschamps AM, Murphy E, Sun J. Estrogen receptor activation and cardioprotection in ischemia reperfusion injury. Trends Cardiovasc Med. 2011;20(3):73–8.
Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109(21 suppl 1):II-2-10.
Gasparyan AY. Cardiovascular risk and inflammation: pathophysiological mechanisms, drug design, and targets. Curr Pharm Des. 2012;18(11):1447–9.
Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin Immunol. 2000;12(1):85–98.
Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53(1):31–47.
Ruparelia N, Digby JE, Andrew J, Debra J, et al. Myocardial infarction causes inflammation and leukocyte recruitment ar remote sites in the myocardium and in the renal glomerulus. Inflamm Res. 2013;62(5):515–25.
Zheng W, Huang LZ, Zhao L, Wang B, Xu HB, Wang GY, et al. Superoxide dismutase activity and malondialdehyde level in plasma and morphological evaluation of acute severe hemorrhagic shock in rats. Am J Emerg Med. 2008;26(1):54–8.
Acknowledgement
This work was supported by National Institute of Pharmaceutical Education and Research-Guwahati, under the aegis of Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Government of India. The authors would like to thank Professor Dr. Ena Dowerah Department of Pathology, Gauhati Medical College, Guwahati, Assam, for evaluation of histopathology slides.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Responsible Editor: John Di Battista.
Rights and permissions
About this article
Cite this article
Kumar, M., Kasala, E.R., Bodduluru, L.N. et al. Baicalein protects isoproterenol induced myocardial ischemic injury in male Wistar rats by mitigating oxidative stress and inflammation. Inflamm. Res. 65, 613–622 (2016). https://doi.org/10.1007/s00011-016-0944-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-016-0944-z