Abstract
Retinoic acid (RA) is of major importance during vertebrate embryonic development and its levels need to be strictly regulated otherwise congenital malformations will develop. Through the action of specific nuclear receptors, named RAR/RXR, RA regulates the expression of genes that eventually influence proliferation and tissue patterning. RA has been described as crucial for different stages of mammalian lung morphogenesis, and as part of a complex molecular network that contributes to precise organogenesis; nonetheless, nothing is known about its role in avian lung development. The current report characterizes, for the first time, the expression pattern of RA signaling members (stra6, raldh2, raldh3, cyp26a1, rarα, and rarβ) and potential RA downstream targets (sox2, sox9, meis1, meis2, tgfβ2, and id2) by in situ hybridization. In the attempt of unveiling the role of RA in chick lung branching, in vitro lung explants were performed. Supplementation studies revealed that RA stimulates lung branching in a dose-dependent manner. Moreover, the expression levels of cyp26a1, sox2, sox9, rarβ, meis2, hoxb5, tgfβ2, id2, fgf10, fgfr2, and shh were evaluated after RA treatment to disclose a putative molecular network underlying RA effect. In situ hybridization analysis showed that RA is able to alter cyp26a1, sox9, tgfβ2, and id2 spatial distribution; to increase rarβ, meis2, and hoxb5 expression levels; and has a very modest effect on sox2, fgf10, fgfr2, and shh expression levels. Overall, these findings support a role for RA in the proximal–distal patterning and branching morphogenesis of the avian lung and reveal intricate molecular interactions that ultimately orchestrate branching morphogenesis.









Similar content being viewed by others
References
Maina JN (2006) Development, structure, and function of a novel respiratory organ, the lung-air sac system of birds: to go where no other vertebrate has gone. Biol Rev 81:545–579. doi:10.1017/S1464793106007111
Metzger RJ, Klein OD, Martin GR, Krasnow MA (2008) The branching programme of mouse lung development. Nature 453(7196):745–750. doi:10.1038/nature07005
Sakiyama J, Yamagishi A, Kuroiwa A (2003) Tbx4-Fgf10 system controls lung bud formation during chicken embryonic development. Development 130(7):1225–1234. doi:10.1242/dev.00345
Moura RS, Coutinho-Borges JP, Pacheco AP, daMota PO, Correia-Pinto J (2011) FGF signaling pathway in the developing chick lung: expression and inhibition studies. PLoS One 6(3):e17660. doi:10.1371/journal.pone.0017660
Moura RS, Carvalho-Correia E, daMota P, Correia-Pinto J (2014) Canonical Wnt signaling activity in early stages of chick lung development. PLoS One 9(12):e112388. doi:10.1371/journal.pone.0112388
Loscertales M, Mikels AJ, Hu JK, Donahoe PK, Roberts DJ (2008) Chick pulmonary Wnt5a directs airway and vascular tubulogenesis. Development 135(7):1365–1376. doi:10.1242/dev.010504
Moura RS, Silva-Gonçalves C, Vaz-Cunha P, Correia-Pinto J (2016) Expression analysis of Shh signaling members in early stages of chick lung development. Histochem Cell Biol 146(4):457–466. doi:10.1007/s00418-016-1448-1
Davey MG, McTeir L, Barrie AM, Freem LJ, Stephen LA (2014) Loss of cilia causes embryonic lung hypoplasia, liver fibrosis, and cholestasis in the talpid3 ciliopathy mutant. Organogenesis 10(2):177–185. doi:10.4161/org.28819
Moura RS, Vaz-Cunha P, Silva-Gonçalves C, Correia-Pinto J (2015) Characterization of miRNA processing machinery in the embryonic chick lung. Cell Tissue Res 362(3):569–575. doi:10.1007/s00441-015-2240-6
Gleghorn JP, Kwak J, Pavlovich AL, Nelson CM (2012) Inhibitory morphogens and monopodial branching of the embryonic chicken lung. Dev Dyn 241(5):852–862. doi:10.1002/dvdy.23771
Ornitz DM, Yin Y (2012) Signaling networks regulating development of the lower respiratory tract. Cold Spring Harb Perspect Biol 4(5):a008318. doi:10.1101/cshperspect.a008318
Malpel S, Mendelsohn C, Cardoso WV (2000) Regulation of retinoic acid signaling during lung morphogenesis. Development 127(14):3057–3067
Piairo P, Moura RS, Nogueira-Silva Correia-Pinto J (2011) The apelinergic system in the developing lung: expression and signaling. Peptides 32(12):2474–2483. doi:10.1016/j.peptides.2011.10.010
Nogueira-Silva C, Piairo P, Carvalho-Dias E, Peixoto FO, Moura RS, Correia-Pinto J (2012) Leukemia inhibitory factor in rat fetal lung development: expression and functional studies. PLoS One 7(1):e30517. doi:10.1371/journal.pone.0030517
Rhinn M, Dollé P (2012) Retinoic acid signalling during development. Development 139(5):843–858. doi:10.1242/dev.065938
Cunningham TJ, Duester G (2015) Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat Rev Mol Cell Biol 16(2):110–123. doi:10.1038/nrm3932
Clagett-Dame M, Knutson D (2011) Vitamin A in reproduction and development. Nutrients 3(4):385–428. doi:10.3390/nu3040385
Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H (2007) A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 315(5813):820–825. doi:10.1126/science.1136244
Ross AC, Zolfaghari R (2011) Cytochrome P450s in the regulation of cellular retinoic acid metabolism. Annu Rev Nutr 31:65–87. doi:10.1146/annurev-nutr-072610-145127
Duester G (2008) Retinoic acid synthesis and signaling during early organogenesis. Cell 134(6):921–931. doi:10.1016/j.cell.2008.09.002
Wilson JG, Roth CB, Warkany J (1953) An analysis of the syndrome of malformations induced by maternal vitamin A deficiency. Effects of restoration of vitamin A at various times during gestation. Am J Anat 92(2):189–217. doi:10.1002/aja.1000920202
Shenefelt RE (1972) Morphogenesis of malformations in hamsters caused by retinoic acid: relation to dose and stage at treatment. Teratology 5:103–118. doi:10.1002/tera.1420050115
Mendelsohn C, Lohnes D, Décimo D, Lufkin T, LeMeur M, Chambon P, Mark M (1994) Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development 120:2749–2771
Niederreither K, Subbarayan V, Dollé P, Chambon P (1999) Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet 21:444–448. doi:10.1038/7788
Niederreither K, Abu-Abed S, Schuhbaur B, Petkovich M, Chambon P, Dollé P (2002) Genetic evidence that oxidative derivatives of retinoic acid are not involved in retinoid signaling during mouse development. Nat Genet 31:84–88. doi:10.1038/ng876
Wang Z, Dollé P, Cardoso WV, Niederreither K (2006) Retinoic acid regulates morphogenesis and patterning of posterior foregut derivatives. Dev Biol 297:433–445. doi:10.1016/j.ydbio.2006.05.019
Chen F, Cao Y, Qian J, Shao F, Niederreither K, Cardoso WV (2010) A retinoic acid-dependent network in the foregut controls formation of the mouse lung primordium. J Clin Investig 120(6):2040–2048. doi:10.1172/JCI40253
Rankin SA, Han L, McCracken KW, Kenny AP, Anglin CT, Grigg EA, Crawford CM, Wells JM, Shannon JM, Zorn AM (2016) A retinoic acid-hedgehog cascade coordinates mesoderm-inducing signals and endoderm competence during lung specification. Cell Rep 16(1):66–78. doi:10.1016/j.celrep.2016.05.060
Chazaud C, Dollé P, Rossant J, Mollard R (2003) Retinoic acid signaling regulates murine bronchial tubule formation. Mech Dev 120(6):691–700. doi:10.1016/S0925-4773(03)00048-0
Chen F, Marquez H, Kim YK, Qian J, Shao F, Fine A, Cruikshank WW, Quadro L, Cardoso WV (2014) Prenatal retinoid deficiency leads to airway hyperresponsiveness in adult mice. J Clin Investig 124:801–811. doi:10.1172/JCI70291
Hind M, Gilthorpe A, Stinchcombe S, Maden M (2009) Retinoid induction of alveolar regeneration: from mice to man? Thorax 64(5):451–457. doi:10.1136/thx.2008.105437
Reijntjes S, Zile MH, Maden M (2010) The expression of Stra6 and Rdh10 in the avian embryo and their contribution to the generation of retinoid signatures. Int J Dev Biol 54(8–9):1267–1275. doi:10.1387/ijdb.093009sr
Tsukui T, Capdevila J, Tamura K, Ruiz-Lozano P, Rodriguez-Esteban C, Yonei-Tamura S, Magallón J, Chandraratna RA, Chien K, Blumberg B, Evans RM, Belmonte JC (1999) Multiple left-right asymmetry defects in Shh(−/−) mutant mice unveil a convergence of the shh and retinoic acid pathways in the control of Lefty-1. Proc Natl Acad Sci USA 96(20):11376–11381. doi:10.1073/pnas.96.20.11376
Sánchez-Guardado LO, Ferran JL, Mijares J, Puelles L, Rodríguez-Gallardo L, Hidalgo-Sánchez M (2009) Raldh3 gene expression pattern in the developing chicken inner ear. J Comp Neurol 514(1):49–65. doi:10.1002/cne.21984
Michaille JJ, Kanzler B, Blanchet S, Garnier JM, Dhouailly D (1995) Characterization of cDNAs encoding two chick retinoic acid receptor alpha isoforms and distribution of retinoic acid receptor alpha, beta and gamma transcripts during chick skin development. Int J Dev Biol 39(4):587–596
Bayha E, Jørgensen MC, Serup P, Grapin-Botton A (2009) Retinoic acid signaling organizes endodermal organ specification along the entire antero-posterior axis. PLoS One 4(6):e5845. doi:10.1371/journal.pone.0005845
Heine P, Dohle E, Bumsted-O’Brien K, Engelkamp D, Schulte D (2008) Evidence for an evolutionary conserved role of homothorax/Meis1/2 during vertebrate retina development. Development 135(5):805–811. doi:10.1242/dev.012088
Gouveia A, Marcelino HM, Gonçalves L, Palmeirim I, Andrade RP (2015) Patterning in time and space: hoxB cluster gene expression in the developing chick embryo. Cell Cycle 14(1):135–145. doi:10.4161/15384101.2014.972868
Yamagishi T, Ando K, Nakamura H, Nakajima Y (2012) Expression of the Tgfβ2 gene during chick embryogenesis. Anat Rec (Hoboken) 295(2):257–267. doi:10.1002/ar.22400
Dady A, Blavet C, Duband JL (2012) Timing and kinetics of E- to N-cadherin switch during neurulation in the avian embryo. Dev Dyn 241(8):1333–1349. doi:10.1002/dvdy.23813
Domowicz MS, Henry JG, Wadlington N, Navarro A, Kraig RP, Schwartz NB (2011) Astrocyte precursor response to embryonic brain injury. Brain Res 1389:35–49. doi:10.1016/j.brainres.2011.03.006
Nakazawa F, Nagai H, Shin M, Sheng G (2006) Negative regulation of primitive hematopoiesis by the FGF signaling pathway. Blood 108(10):3335–3343. doi:10.1182/blood-2006-05-021386
Riddle RD, Johnson RL, Laufer E, Tabin C (1993) Sonic hedgehog mediates the polarizing activity of the ZPA. Cell 75(7):1401–1416. doi:10.1016/0092-8674(93)90626-2
Lorda-Diez CI, Torre-Pérez N, García-Porrero JA, Hurle JM, Montero JA (2009) Expression of Id2 in the developing limb is associated with zones of active BMP signaling and marks the regions of growth and differentiation of the developing digits. Int J Dev Biol 53(8–10):1495–1502. doi:10.1387/ijdb.072415cl
Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D (1995) Expression of a Delta homologue in prospective neurons in the chick. Nature 375:787–790. doi:10.1038/375787a0
Zachman RD (1995) Role of vitamin A in lung development. J Nutr 125(6 Suppl):1634S–1638S
Swarr DT, Morrisey EE (2015) Lung endoderm morphogenesis: gasping for form and function. Annu Rev Cell Dev Biol 31:553–573. doi:10.1146/annurev-cellbio-100814-125249
Fex G, Johannesson G (1988) Retinol transfer across and between phospholipid bilayer membranes. Biochim Biophys Acta 944(2):249–255. doi:10.1016/0005-2736(88)90438-5
Bouillet P, Sapin V, Chazaud C, Messaddeq N, Décimo D, Dollé P, Chambon P (1997) Developmental expression pattern of Stra6, a retinoic acid-responsive gene encoding a new type of membrane protein. Mech Dev 63(2):173–186. doi:10.1016/S0925-4773(97)00039-7
Golzio C, Martinovic-Bouriel J, Thomas S, Mougou-Zrelli S, Grattagliano-Bessieres B, Bonniere M, Delahaye S, Munnich A, Encha-Razavi F, Lyonnet S, Vekemans M, Attie-Bitach T, Etchevers HC (2007) Matthew-Wood syndrome is caused by truncating mutations in the retinol-binding protein receptor gene STRA6. Am J Hum Genet 80(6):1179–1187. doi:10.1086/518177
Pasutto F, Sticht H, Hammersen G, Gillessen-Kaesbach G, Fitzpatrick DR, Nürnberg G, Brasch F, Schirmer-Zimmermann H, Tolmie JL, Chitayat D, Houge G, Fernández-Martínez L, Keating S, Mortier G, Hennekam RC, von der Wense A, Slavotinek A, Meinecke P, Bitoun P, Becker C, Nürnberg P, Reis A, Rauch A (2007) Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am J Hum Genet 80(3):550–560. doi:10.1086/512203
Miura T, Hartmann D, Kinboshi M, Komada M, Ishibashi M, Shiota K (2009) The cyst-branch difference in developing chick lung results from a different morphogen diffusion coefficient. Mech Dev 126(3–4):160–172. doi:10.1016/j.mod.2008.11.006
Dupé V, Matt N, Garnier JM, Chambon P, Mark M, Ghyselinck NB (2003) A newborn lethal defect due to inactivation of retinaldehyde dehydrogenase type 3 is prevented by maternal retinoic acid treatment. Proc Natl Acad Sci USA 100(24):14036–14041. doi:10.1073/pnas.2336223100
Li E, Sucov HM, Lee KF, Evans RM, Jaenisch R (1993) Normal development and growth of mice carrying a targeted disruption of the alpha 1 retinoic acid receptor gene. Proc Natl Acad Sci USA 15(90):1590–1594
Luo J, Pasceri P, Conlon RA, Rossant J, Giguère V (1995) Mice lacking all isoforms of retinoic acid receptor beta develop normally and are susceptible to the teratogenic effects of retinoic acid. Mech Dev 53(1):61–71. doi:10.1016/0925-4773(95)00424-6
Lohnes D, Mark M, Mendelsohn C, Dollé P, Dierich A, Gorry P, Gansmuller A, Chambon P (1994) Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development 120(10):2723–2748
Dollé P, Rubert E, Leroy P, Morriss-Kay G, Chambon P (1990) Retinoic acid receptors and cellular retinoid binding proteins I. A systematic study of their differential pattern of transcription during mouse organogenesis. Development 110:1133–1151
Mollard R, Ghyselinck NB, Wendling O, Chambon P, Mark M (2000) Stage-dependent responses of the developing lung to retinoic acid signaling. Int J Dev Biol 44(5):457–462
Mollard R, Viville S, Ward SJ, Décimo D, Chambon P, Dollé P (2000) Tissue-specific expression of retinoic acid receptor isoform transcripts in the mouse embryo. Mech Dev 94(1–2):223–232. doi:10.1016/S0925-4773(00)00303-8
Mercader N, Leonardo E, Piedra ME, Martínez-A C, Ros MA, Torres M (2000) Opposing RA and FGF signals control proximodistal vertebrate limb development through regulation of Meis genes. Development 127(18):3961–3970
Liu J, Tseu I, Wang J, Tanswell K, Post M (2000) Transforming growth factor beta2, but not beta1 and beta3, is critical for early rat lung branching. Dev Dyn 217(4):343–360. doi:10.1002/(SICI)1097-0177(200004)217:4<343:AID-DVDY2>3.0.CO;2-F
Gontan C, de Munck A, Vermeij M, Grosveld F, Tibboel D, Rottier R (2008) Sox2 is important for two crucial processes in lung development: branching morphogenesis and epithelial cell differentiation. Dev Biol 317(1):296–309. doi:10.1016/j.ydbio.2008.02.035
Rockich BE, Hrycaj SM, Shih HP, Nagy MS, Ferguson MA, Kopp JL, Sander M, Wellik DM, Spence JR (2013) Sox9 plays multiple roles in the lung epithelium during branching morphogenesis. Proc Natl Acad Sci USA 110(47):E4456–E4464. doi:10.1073/pnas.1311847110
Kamachi Y, Kondoh H (2013) Sox proteins: regulators of cell fate specification and differentiation. Development 140(20):4129–4144. doi:10.1242/dev.091793
Zhu Y, Li Y, Jun Wei JW, Liu X (2012) The role of Sox genes in lung morphogenesis and cancer. Int J Mol Sci 13(12):15767–15783. doi:10.3390/ijms131215767
Ishii Y, Rex M, Scotting PJ, Yasugi S (1998) Region-specific expression of chicken Sox2 in the developing gut and lung epithelium: regulation by epithelial–mesenchymal interactions. Dev Dyn 213(4):464–475. doi:10.1002/(SICI)1097-0177(199812)213:4<464:AID-AJA11>3.0.CO;2-Z
Que J, Okubo T, Goldenring JR, Nam KT, Kurotani R, Morrisey EE, Taranova O, Pevny LH, Hogan BL (2007) Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development 134(13):2521–2531. doi:10.1242/dev.003855
Que J, Luo X, Schwartz RJ, Hogan BL (2009) Multiple roles for Sox2 in the developing and adult mouse trachea. Development 136(11):1899–1907. doi:10.1242/dev.034629
Tompkins DH, Besnard V, Lange AW, Keiser AR, Wert SE, Bruno MD, Whitsett JA (2011) Sox2 activates cell proliferation and differentiation in the respiratory epithelium. Am J Respir Cell Mol Biol 45(1):101–110. doi:10.1165/rcmb.2010-0149OC
Liu Y, Hogan BL (2002) Differential gene expression in the distal tip endoderm of the embryonic mouse lung. Gene Expr Patterns 2(3–4):229–233. doi:10.1016/S1567-133X(02)00057-1
Turcatel G, Rubin N, Menke DB, Martin G, Shi W, Warburton D (2013) Lung mesenchymal expression of Sox9 plays a critical role in tracheal development. BMC Biol 11:117. doi:10.1186/1741-7007-11-117
Park J, Zhang JJ, Moro A, Kushida M, Wegner M, Kim PC (2010) Regulation of Sox9 by Sonic Hedgehog (Shh) is essential for patterning and formation of tracheal cartilage. Dev Dyn 239(2):514–526. doi:10.1002/dvdy.22192
Turcatel G, Millette K, Thornton M, Leguizamon S, Grubbs B, Shi W, Warburton D (2017) Cartilage rings contribute to the proper embryonic tracheal epithelial differentiation, metabolism, and expression of inflammatory genes. Am J Physiol Lung Cell Mol Physiol 312(2):L196–L207. doi:10.1152/ajplung.00127.2016
Rawlins EL, Clark CP, Xue Y, Hogan BL (2009) The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development 136(22):3741–3745. doi:10.1242/dev.037317
Marklund M, Sjödal M, Beehler BC, Jessell TM, Edlund T, Gunhaga L (2004) Retinoic acid signalling specifies intermediate character in the developing telencephalon. Development 131(17):4323–4332. doi:10.1242/dev.01308
Capdevila J, Tsukui T, Rodríquez Esteban C, Zappavigna V, Izpisúa Belmonte JC (1999) Control of vertebrate limb outgrowth by the proximal factor Meis2 and distal antagonism of BMPs by Gremlin. Mol Cell 4(5):839–849. doi:10.1016/S1097-2765(00)80393-7
Pelton RW, Dickinson ME, Moses HL, Hogan BL (1990) In situ hybridization analysis of TGF beta 3 RNA expression during mouse development: comparative studies with TGF beta 1 and beta 2. Development 110(2):609–620
Bragg AD, Moses HL, Serra R (2001) Signaling to the epithelium is not sufficient to mediate all of the effects of transforming growth factor beta and bone morphogenetic protein 4 on murine embryonic lung development. Mech Dev 109(1):13–26. doi:10.1016/S0925-4773(01)00508-1
Tang XH, Gudas LJ (2011) Retinoids, retinoic acid receptors, and cancer. Annu Rev Pathol 6:345–364. doi:10.1146/annurev-pathol-011110-130303
Maden M (2004) Retinoids in lung development and regeneration. Curr Topics Dev Biol 61:153–189. doi:10.1016/S0070-2153(04)61007-6
Cardoso WV, Williams MC, Mitsialis SA, Joyce-Brady M, Rishi AK, Brody JS (1995) Retinoic acid induces changes in the pattern of airway branching and alters epithelial cell differentiation in the developing lung in vitro. Am J Respir Cell Mol Biol 12(5):464–476. doi:10.1165/ajrcmb.12.5.7742011
Pereira-Terra P, Moura RS, Nogueira-Silva C, Correia-Pinto J (2015) Neuroendocrine factors regulate retinoic acid receptors in normal and hypoplastic lung development. J Physiol 593(15):3301–3311. doi:10.1113/JP270477
Schuger L, Varani J, Mitra R Jr, Gilbride K (1993) Retinoic acid stimulates mouse lung development by a mechanism involving epithelial–mesenchymal interaction and regulation of epidermal growth factor receptors. Dev Biol 159(2):462–473. doi:10.1006/dbio.1993.1256
Packer AI, Mailutha KG, Ambrozewicz LA, Wolgemuth DJ (2000) Regulation of the Hoxa4 and Hoxa5 genes in the embryonic mouse lung by retinoic acid and TGFbeta1: implications for lung development and patterning. Dev Dyn 217(1):62–74. doi:10.1002/(SICI)1097-0177(200001)217:1<62:AID-DVDY6>3.0.CO;2-U
Volpe MV, Vosatka RJ, Nielsen HC (2000) Hoxb-5 control of early airway formation during branching morphogenesis in the developing mouse lung. Biochim Biophys Acta 1475(3):337–345. doi:10.1016/S0304-4165(00)00087-8
Chang DR, Martinez Alanis D, Miller RK, Ji H, Akiyama H, McCrea PD, Chen J (2013) Lung epithelial branching program antagonizes alveolar differentiation. Proc Natl Acad Sci USA 110(45):18042–18051. doi:10.1073/pnas.1311760110
Bürglin TR, Affolter M (2016) Homeodomain proteins: an update. Chromosoma 125(3):497–521. doi:10.1007/s00412-015-0543-8
Wellik DM (2007) Hox patterning of the vertebrate axial skeleton. Dev Dyn 236(9):2454–2463. doi:10.1002/dvdy.21286
Sakiyama J, Yokouchi Y, Kuroiwa A (2000) Coordinated expression of Hoxb genes and signaling molecules during development of the chick respiratory tract. Dev Biol 227(1):12–27. doi:10.1006/dbio.2000.9880
Cardoso WV, Mitsialis SA, Brody JS, Williams MC (1996) Retinoic acid alters the expression of pattern-related genes in the developing rat lung. Dev Dyn 207(1):47–59. doi:10.1002/(SICI)1097-0177(199609)207:1<47:AID-AJA6>3.0.CO;2-W
Volpe MV, Ramadurai SM, Pham LD, Nielsen HC (2007) Hoxb-5 down regulation alters Tenascin-C, FGF10 and Hoxb gene expression patterns in pseudoglandular period fetal mouse lung. Front Biosci 12:860–873. doi:10.2741/2108
Chen F, Desai TJ, Qian J, Niederreither K, Lü J, Cardoso WV (2007) Inhibition of Tgf beta signaling by endogenous retinoic acid is essential for primary lung bud induction. Development 134(16):2969–2979. doi:10.1242/dev.006221
Chen H, Zhuang F, Liu YH, Xu B, Del Moral P, Deng W, Chai Y, Kolb M, Gauldie J, Warburton D, Moses HL, Shi W (2008) TGF-beta receptor II in epithelia versus mesenchyme plays distinct roles in the developing lung. Eur Respir J 32(2):285–295. doi:10.1183/09031936.00165407
Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J (1995) Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial–mesenchymal interaction. Nat Genet 11(4):415–421. doi:10.1038/ng1295-415
Serra R, Pelton RW, Moses HL (1994) TGF beta 1 inhibits branching morphogenesis and N-myc expression in lung bud organ cultures. Development 120(8):2153–2161
Zhao J, Bu D, Lee M, Slavkin HC, Hall FL, Warburton D (1996) Abrogation of transforming growth factor-beta type II receptor stimulates embryonic mouse lung branching morphogenesis in culture. Dev Biol 180(1):242–257. doi:10.1006/dbio.1996.0298
Roschger C, Cabrele C (2017) The Id-protein family in developmental and cancer-associated pathways. Cell Commun Signal 15(1):7. doi:10.1186/s12964-016-0161-y
Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, Yagishita N, Matsui D, Koga Y, Itoh N, Kato S (1999) Fgf10 is essential for limb and lung formation. Nat Genet 21(1):138–141. doi:10.1038/5096
Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL (1997) Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development 124(23):4867–4878
Fernandes-Silva H, Correia-Pinto J, Moura RS (2017) Canonical sonic hedgehog signaling in early lung development. J Dev Biol 5(1):3. doi:10.3390/jdb5010003
Acknowledgements
The authors would like to thank Ana Lima for slide sectioning and Rita Lopes for contributing to the initiation of this project. This work has been funded by FEDER funds, through the Competitiveness Factors Operational Programme (COMPETE), and by National funds, through the Foundation for Science and Technology (FCT), under the scope of the Project POCI-01-0145-FEDER-007038; and by the Project NORTE-01-0145-FEDER-000013, supported by the Northern Portugal Regional Operational Programme (NORTE 2020), under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (FEDER). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
18_2017_2600_MOESM1_ESM.tif
Fig. S1 Hematoxylin and eosin staining of slide sections corresponding to stra6 (a), raldh2 (b), cyp26a1 (c), rarα (d) and rarβ (e) represented in Fig. 1. Scale bar: 100 µm. (TIFF 9236 kb)
18_2017_2600_MOESM2_ESM.tif
Fig. S2 Hematoxylin and eosin staining of slide sections corresponding to sox2 (a), sox9 (b), meis1 (c), meis2 (d) and tgfβ2 (e) represented in Fig. 2. Scale bar: 100 µm. (TIFF 9228 kb)
18_2017_2600_MOESM3_ESM.tif
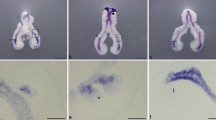
Fig. S3 l-cam expression pattern at early stages of chick lung development. Representative examples of in situ hybridization of stage b1, b2 and b3 lungs for l-cam (a-d), n ≥ 6 per stage. l-cam is exclusively expressed in the pulmonary epithelium (b, d: black arrow) and the secondary bronchi (c, d: asterisk). Scale bar: whole mount, 500 µm; slide section, 100 µm. The black rectangle in image c indicate the region shown in corresponding slide section. (TIFF 3926 kb)
18_2017_2600_MOESM4_ESM.tif
Fig. S4 id2 expression pattern at early stages of chick lung development. Representative examples of in situ hybridization of stage b1, b2 and b3 lungs for id2 (a-d), n ≥ 6 per stage. id2 is present in the mesenchymal compartment, mainly in ventral region (b; open arrowhead) and, specifically, in the dorsal region surrounding the proximal-most bud (c; dashed arrow). Expression around the trachea (a; dark arrowhead) seems to be stage dependent. Epithelial expression seems to be restricted to the growing tips (b; asterisk). Slide sectioning revealed that id2 is absent from the epithelium of the main bronchi (d; black arrow), but it is expressed in the distal ends of secondary bronchi (d; asterisk). Dorsal mesenchyme was identified as mesothelium (d; section sign) in the slide sections. Scale bar: whole mount, 500 µm; slide section, 100 µm. The black rectangle in image c indicates the region shown in corresponding slide section. (TIFF 3972 kb)
Rights and permissions
About this article
Cite this article
Fernandes-Silva, H., Vaz-Cunha, P., Barbosa, V.B. et al. Retinoic acid regulates avian lung branching through a molecular network. Cell. Mol. Life Sci. 74, 4599–4619 (2017). https://doi.org/10.1007/s00018-017-2600-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2600-3