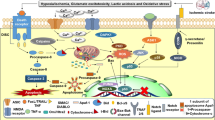
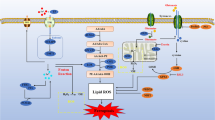
Abstract
Apoptosis, necrosis and autophagy-dependent cell death are the three major types of cell death. Traditionally, necrosis is thought as a passive and unregulated form of cell death. However, certain necrosis can also occur in a highly regulated manner, referring to regulated necrosis. Depending on the signaling pathways, regulated necrosis can be further classified as necroptosis, pyroptosis, ferroptosis, parthanatos and CypD-mediated necrosis. Numerous studies have reported that regulated necrosis contributes to the progression of multiple injury-relevant diseases. For example, necroptosis contributes to the development of myocardial infarction, atherosclerosis, heart failure and stroke; pyroptosis is involved in the progression of myocardial or cerebral infarction, atherosclerosis and diabetic cardiomyopathy; while ferroptosis, parthanatos and CypD-mediated necrosis participate in the pathological process of myocardial and/or cerebral ischemia/reperfusion injury. Thereby, targeting the pathways of regulated necrosis pharmacologically or genetically could be an efficient strategy for reducing cardio-cerebrovascular injury. Further study needs to focus on the crosstalk and interplay among different types of regulated necrosis. Pharmacological intervention of two or more types of regulated necrosis simultaneously may have advantages in clinic to treat injury-relevant diseases.



Similar content being viewed by others
References
Grootjans S, Vanden Berghe T, Vandenabeele P (2017) Initiation and execution mechanisms of necroptosis: an overview. Cell Death Differ 24(7):1184–1195. https://doi.org/10.1038/cdd.2017.65
Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J (2000) Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 1(6):489–495. https://doi.org/10.1038/82732
Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK (2009) Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137(6):1112–1123. https://doi.org/10.1016/j.cell.2009.05.037
Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez-Diaz S, Lewis R, Lalaoui N, Metcalf D, Webb AI, Young SN, Varghese LN, Tannahill GM, Hatchell EC, Majewski IJ, Okamoto T, Dobson RC, Hilton DJ, Babon JJ, Nicola NA, Strasser A, Silke J, Alexander WS (2013) The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 39(3):443–453. https://doi.org/10.1016/j.immuni.2013.06.018
Latz E, Xiao TS, Stutz A (2013) Activation and regulation of the inflammasomes. Nat Rev Immunol 13(6):397–411. https://doi.org/10.1038/nri3452
Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, Schroder GF, Fitzgerald KA, Wu H, Egelman EH (2014) Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 156(6):1193–1206. https://doi.org/10.1016/j.cell.2014.02.008
Shi J, Gao W, Shao F (2017) Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci 42(4):245–254. https://doi.org/10.1016/j.tibs.2016.10.004
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072. https://doi.org/10.1016/j.cell.2012.03.042
Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR (2016) Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci USA 113(34):E4966–E4975. https://doi.org/10.1073/pnas.1603244113
Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL (2002) Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 297(5579):259–263. https://doi.org/10.1126/science.1072221
Yu SW, Andrabi SA, Wang H, Kim NS, Poirier GG, Dawson TM, Dawson VL (2006) Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc Natl Acad Sci USA 103(48):18314–18319. https://doi.org/10.1073/pnas.0606528103
Wang Y, Kim NS, Haince JF, Kang HC, David KK, Andrabi SA, Poirier GG, Dawson VL, Dawson TM (2011) Poly(ADP-ribose) (PAR) binding to apoptosis-inducing factor is critical for PAR polymerase-1-dependent cell death (parthanatos). Sci Signal 4(167):20. https://doi.org/10.1126/scisignal.2000902
Fatokun AA, Dawson VL, Dawson TM (2014) Parthanatos: mitochondrial-linked mechanisms and therapeutic opportunities. Br J Pharmacol 171(8):2000–2016. https://doi.org/10.1111/bph.12416
Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD (2005) Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434(7033):658–662. https://doi.org/10.1038/nature03434
Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y (2005) Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434(7033):652–658. https://doi.org/10.1038/nature03317
Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ (2005) Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci USA 102(34):12005–12010. https://doi.org/10.1073/pnas.0505294102
Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P (2014) Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol 15(2):135–147. https://doi.org/10.1038/nrm3737
Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, Baehrecke EH, Barlev NA, Bazan NG, Bernassola F, Bertrand MJM, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Boya P, Brenner C, Campanella M, Candi E, Carmona-Gutierrez D, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Cohen GM, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D’Angiolella V, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin KM, DeBerardinis RJ, Deshmukh M, Di Daniele N, Di Virgilio F, Dixit VM, Dixon SJ, Duckett CS, Dynlacht BD, El-Deiry WS, Elrod JW, Fimia GM, Fulda S, Garcia-Saez AJ, Garg AD, Garrido C, Gavathiotis E, Golstein P, Gottlieb E, Green DR, Greene LA, Gronemeyer H, Gross A, Hajnoczky G, Hardwick JM, Harris IS, Hengartner MO, Hetz C, Ichijo H, Jaattela M, Joseph B, Jost PJ, Juin PP, Kaiser WJ, Karin M, Kaufmann T, Kepp O, Kimchi A, Kitsis RN, Klionsky DJ, Knight RA, Kumar S, Lee SW, Lemasters JJ, Levine B, Linkermann A, Lipton SA, Lockshin RA, Lopez-Otin C, Lowe SW, Luedde T, Lugli E, MacFarlane M, Madeo F, Malewicz M, Malorni W, Manic G, Marine JC, Martin SJ, Martinou JC, Medema JP, Mehlen P, Meier P, Melino S, Miao EA, Molkentin JD, Moll UM, Munoz-Pinedo C, Nagata S, Nunez G, Oberst A, Oren M, Overholtzer M, Pagano M, Panaretakis T, Pasparakis M, Penninger JM, Pereira DM, Pervaiz S, Peter ME, Piacentini M, Pinton P, Prehn JHM, Puthalakath H, Rabinovich GA, Rehm M, Rizzuto R, Rodrigues CMP, Rubinsztein DC, Rudel T, Ryan KM, Sayan E, Scorrano L, Shao F, Shi Y, Silke J, Simon HU, Sistigu A, Stockwell BR, Strasser A, Szabadkai G, Tait SWG, Tang D, Tavernarakis N, Thorburn A, Tsujimoto Y, Turk B, Vanden Berghe T, Vandenabeele P, Vander Heiden MG, Villunger A, Virgin HW, Vousden KH, Vucic D, Wagner EF, Walczak H, Wallach D, Wang Y, Wells JA, Wood W, Yuan J, Zakeri Z, Zhivotovsky B, Zitvogel L, Melino G, Kroemer G (2018) Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25(3):486–541. https://doi.org/10.1038/s41418-017-0012-4
Del Re DP, Amgalan D, Linkermann A, Liu Q, Kitsis RN (2019) Fundamental mechanisms of regulated cell death and implications for heart disease. Physiol Rev 99(4):1765–1817. https://doi.org/10.1152/physrev.00022.2018
Dmitriev YV, Minasian SM, Demchenko EA, Galagudza MM (2013) Study of cardioprotective effects of necroptosis inhibitors on isolated rat heart subjected to global ischemia-reperfusion. Bull Exp Biol Med 155(2):245–248. https://doi.org/10.1007/s10517-013-2124-2
Yue LJ, Zhu XY, Li RS, Chang HJ, Gong B, Tian CC, Liu C, Xue YX, Zhou Q, Xu TS, Wang DJ (2019) Sallylcysteine sulfoxide (alliin) alleviates myocardial infarction by modulating cardiomyocyte necroptosis and autophagy. Int J Mol Med 44(5):1943–1951. https://doi.org/10.3892/ijmm.2019.4351
Bai J, Wang Q, Qi J, Yu H, Wang C, Wang X, Ren Y, Yang F (2019) Promoting effect of baicalin on nitric oxide production in CMECs via activating the PI3K-AKT-eNOS pathway attenuates myocardial ischemia-reperfusion injury. Phytomedicine 63:153035. https://doi.org/10.1016/j.phymed.2019.153035
Luedde M, Lutz M, Carter N, Sosna J, Jacoby C, Vucur M, Gautheron J, Roderburg C, Borg N, Reisinger F, Hippe HJ, Linkermann A, Wolf MJ, Rose-John S, Lullmann-Rauch R, Adam D, Flogel U, Heikenwalder M, Luedde T, Frey N (2014) RIP3, a kinase promoting necroptotic cell death, mediates adverse remodelling after myocardial infarction. Cardiovasc Res 103(2):206–216. https://doi.org/10.1093/cvr/cvu146
Newton K, Dugger DL, Maltzman A, Greve JM, Hedehus M, Martin-McNulty B, Carano RA, Cao TC, van Bruggen N, Bernstein L, Lee WP, Wu X, DeVoss J, Zhang J, Jeet S, Peng I, McKenzie BS, Roose-Girma M, Caplazi P, Diehl L, Webster JD, Vucic D (2016) RIPK3 deficiency or catalytically inactive RIPK1 provides greater benefit than MLKL deficiency in mouse models of inflammation and tissue injury. Cell Death Differ 23(9):1565–1576. https://doi.org/10.1038/cdd.2016.46
Ghardashi Afousi A, Gaeini A, Rakhshan K, Naderi N, Darbandi Azar A, Aboutaleb N (2019) Targeting necroptotic cell death pathway by high-intensity interval training (HIIT) decreases development of post-ischemic adverse remodelling after myocardial ischemia/reperfusion injury. J Cell Commun Signal 13(2):255–267. https://doi.org/10.1007/s12079-018-0481-3
She L, Tu H, Zhang YZ, Tang LJ, Li NS, Ma QL, Liu B, Li Q, Luo XJ, Peng J (2019) Inhibition of phosphoglycerate mutase 5 reduces necroptosis in rat hearts following ischemia/reperfusion through suppression of dynamin-related protein 1. Cardiovasc Drugs Ther 33(1):13–23. https://doi.org/10.1007/s10557-018-06848-8
Wang Z, Jiang H, Chen S, Du F, Wang X (2012) The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell 148(1–2):228–243. https://doi.org/10.1016/j.cell.2011.11.030
Kanugula AK, Thodeti CK (2018) AMP-activated kinase “Keaps” ischemia/reperfusion-induced necroptosis under control. Int J Cardiol 259:168–169. https://doi.org/10.1016/j.ijcard.2018.02.053
Wang YS, Yu P, Wang Y, Zhang J, Hang W, Yin ZX, Liu G, Chen J, Werle KD, Quan CS, Gao H, Zeng Q, Cui R, Liang J, Ding Q, Li YL, Xu ZX (2018) AMP-activated protein kinase protects against necroptosis via regulation of Keap1-PGAM5 complex. Int J Cardiol 259:153–162. https://doi.org/10.1016/j.ijcard.2018.01.036
Zhang T, Zhang Y, Cui M, Jin L, Wang Y, Lv F, Liu Y, Zheng W, Shang H, Zhang J, Zhang M, Wu H, Guo J, Zhang X, Hu X, Cao CM, Xiao RP (2016) CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat Med 22(2):175–182. https://doi.org/10.1038/nm.4017
Yang Z, Li C, Wang Y, Yang J, Yin Y, Liu M, Shi Z, Mu N, Yu L, Ma H (2018) Melatonin attenuates chronic pain related myocardial ischemic susceptibility through inhibiting RIP3-MLKL/CaMKII dependent necroptosis. J Mol Cell Cardiol 125:185–194. https://doi.org/10.1016/j.yjmcc.2018.10.018
Zhu P, Hu S, Jin Q, Li D, Tian F, Toan S, Li Y, Zhou H, Chen Y (2018) Ripk3 promotes ER stress-induced necroptosis in cardiac IR injury: a mechanism involving calcium overload/XO/ROS/mPTP pathway. Redox Biol 16:157–168. https://doi.org/10.1016/j.redox.2018.02.019
Zhou H, Li D, Zhu P, Ma Q, Toan S, Wang J, Hu S, Chen Y, Zhang Y (2018) Inhibitory effect of melatonin on necroptosis via repressing the Ripk3-PGAM5-CypD-mPTP pathway attenuates cardiac microvascular ischemia-reperfusion injury. J Pineal Res 65(3):e12503. https://doi.org/10.1111/jpi.12503
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, Writing Group M, American Heart Association Statistics C, Stroke Statistics S (2016) Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 133(4):e38–e360. https://doi.org/10.1161/CIR.0000000000000350
Wang D, Wang Z, Zhang L, Wang Y (2017) Roles of cells from the arterial vessel wall in atherosclerosis. Mediat Inflamm 2017:8135934. https://doi.org/10.1155/2017/8135934
Tian F, Yao J, Yan M, Sun X, Wang W, Gao W, Tian Z, Guo S, Dong Z, Li B, Gao T, Shan P, Liu B, Wang H, Cheng J, Gao Q, Zhang Z, Cao W, Tian Y (2016) 5-Aminolevulinic acid-mediated sonodynamic therapy inhibits RIPK1/RIPK3-dependent necroptosis in THP-1-derived foam cells. Sci Rep 6:21992. https://doi.org/10.1038/srep21992
Lin J, Li H, Yang M, Ren J, Huang Z, Han F, Huang J, Ma J, Zhang D, Zhang Z, Wu J, Huang D, Qiao M, Jin G, Wu Q, Huang Y, Du J, Han J (2013) A role of RIP3-mediated macrophage necrosis in atherosclerosis development. Cell Rep 3(1):200–210. https://doi.org/10.1016/j.celrep.2012.12.012
Meng L, Jin W, Wang X (2015) RIP3-mediated necrotic cell death accelerates systematic inflammation and mortality. Proc Natl Acad Sci USA 112(35):11007–11012. https://doi.org/10.1073/pnas.1514730112
Karunakaran D, Geoffrion M, Wei L, Gan W, Richards L, Shangari P, DeKemp EM, Beanlands RA, Perisic L, Maegdefessel L, Hedin U, Sad S, Guo L, Kolodgie FD, Virmani R, Ruddy T, Rayner KJ (2016) Targeting macrophage necroptosis for therapeutic and diagnostic interventions in atherosclerosis. Sci Adv 2(7):e1600224. https://doi.org/10.1126/sciadv.1600224
Chtourou Y, Slima AB, Makni M, Gdoura R, Fetoui H (2015) Naringenin protects cardiac hypercholesterolemia-induced oxidative stress and subsequent necroptosis in rats. Pharmacol Rep 67(6):1090–1097. https://doi.org/10.1016/j.pharep.2015.04.002
Zhang YZ, Wang L, Zhang JJ, Xiong XM, Zhang D, Tang XM, Luo XJ, Ma QL, Peng J (2018) Vascular peroxide 1 promotes ox-LDL-induced programmed necrosis in endothelial cells through a mechanism involving beta-catenin signaling. Atherosclerosis 274:128–138. https://doi.org/10.1016/j.atherosclerosis.2018.04.031
Szobi A, Goncalvesova E, Varga ZV, Leszek P, Kusmierczyk M, Hulman M, Kyselovic J, Ferdinandy P, Adameova A (2017) Analysis of necroptotic proteins in failing human hearts. J Transl Med 15(1):86. https://doi.org/10.1186/s12967-017-1189-5
Li L, Chen Y, Doan J, Murray J, Molkentin JD, Liu Q (2014) Transforming growth factor beta-activated kinase 1 signaling pathway critically regulates myocardial survival and remodeling. Circulation 130(24):2162–2172. https://doi.org/10.1161/CIRCULATIONAHA.114.011195
Guo X, Yin H, Li L, Chen Y, Li J, Doan J, Steinmetz R, Liu Q (2017) Cardioprotective role of tumor necrosis factor receptor-associated factor 2 by suppressing apoptosis and necroptosis. Circulation 136(8):729–742. https://doi.org/10.1161/CIRCULATIONAHA.116.026240
Zhao M, Lu L, Lei S, Chai H, Wu S, Tang X, Bao Q, Chen L, Wu W, Liu X (2016) Inhibition of receptor interacting protein kinases attenuates cardiomyocyte hypertrophy induced by palmitic acid. Oxid Med Cell Longev 2016:1451676. https://doi.org/10.1155/2016/1451676
Soppert J, Kraemer S, Beckers C, Averdunk L, Mollmann J, Denecke B, Goetzenich A, Marx G, Bernhagen J, Stoppe C (2018) Soluble CD74 reroutes MIF/CXCR4/AKT-mediated survival of cardiac myofibroblasts to necroptosis. J Am Heart Assoc 7(17):e009384. https://doi.org/10.1161/JAHA.118.009384
Peng S, Xu J, Ruan W, Li S, Xiao F (2017) PPAR-gamma activation prevents septic cardiac dysfunction via inhibition of apoptosis and necroptosis. Oxid Med Cell Longev 2017:8326749. https://doi.org/10.1155/2017/8326749
Zhou F, Jiang X, Teng L, Yang J, Ding J, He C (2018) Necroptosis may be a novel mechanism for cardiomyocyte death in acute myocarditis. Mol Cell Biochem 442(1–2):11–18. https://doi.org/10.1007/s11010-017-3188-5
Zhang L, Feng Q, Wang T (2018) Necrostatin-1 protects against paraquat-induced cardiac contractile dysfunction via RIP1-RIP3-MLKL-dependent necroptosis pathway. Cardiovasc Toxicol 18(4):346–355. https://doi.org/10.1007/s12012-017-9441-z
Xu Z, Jin Y, Yan H, Gao Z, Xu B, Yang B, He Q, Shi Q, Luo P (2018) High-mobility group box 1 protein-mediated necroptosis contributes to dasatinib-induced cardiotoxicity. Toxicol Lett 296:39–47. https://doi.org/10.1016/j.toxlet.2018.08.003
Zhang L, Wei J, Ren L, Zhang J, Yang M, Jing L, Wang J, Sun Z, Zhou X (2017) Endosulfan inducing apoptosis and necroptosis through activation RIPK signaling pathway in human umbilical vascular endothelial cells. Environ Sci Pollut Res Int 24(1):215–225. https://doi.org/10.1007/s11356-016-7652-7
Xu Y, Wang J, Song X, Qu L, Wei R, He F, Wang K, Luo B (2016) RIP3 induces ischemic neuronal DNA degradation and programmed necrosis in rat via AIF. Sci Rep 6:29362. https://doi.org/10.1038/srep29362
Yang R, Hu K, Chen J, Zhu S, Li L, Lu H, Li P, Dong R (2017) Necrostatin-1 protects hippocampal neurons against ischemia/reperfusion injury via the RIP3/DAXX signaling pathway in rats. Neurosci Lett 651:207–215. https://doi.org/10.1016/j.neulet.2017.05.016
Li J, Zhang J, Zhang Y, Wang Z, Song Y, Wei S, He M, You S, Jia J, Cheng J (2019) TRAF2 protects against cerebral ischemia-induced brain injury by suppressing necroptosis. Cell Death Dis 10(5):328. https://doi.org/10.1038/s41419-019-1558-5
Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1(2):112–119. https://doi.org/10.1038/nchembio711
Yin B, Xu Y, Wei RL, He F, Luo BY, Wang JY (2015) Inhibition of receptor-interacting protein 3 upregulation and nuclear translocation involved in Necrostatin-1 protection against hippocampal neuronal programmed necrosis induced by ischemia/reperfusion injury. Brain Res 1609:63–71. https://doi.org/10.1016/j.brainres.2015.03.024
Chen Y, Zhang L, Yu H, Song K, Shi J, Chen L, Cheng J (2018) Necrostatin-1 improves long-term functional recovery through protecting oligodendrocyte precursor cells after transient focal cerebral ischemia in mice. Neuroscience 371:229–241. https://doi.org/10.1016/j.neuroscience.2017.12.007
Li W, Liu J, Chen JR, Zhu YM, Gao X, Ni Y, Lin B, Li H, Qiao SG, Wang C, Zhang HL, Ao GZ (2018) Neuroprotective effects of DTIO, a novel analog of Nec-1, in acute and chronic stages after ischemic stroke. Neuroscience 390:12–29. https://doi.org/10.1016/j.neuroscience.2018.07.044
Yang XS, Yi TL, Zhang S, Xu ZW, Yu ZQ, Sun HT, Yang C, Tu Y, Cheng SX (2017) Hypoxia-inducible factor-1 alpha is involved in RIP-induced necroptosis caused by in vitro and in vivo ischemic brain injury. Sci Rep 7(1):5818. https://doi.org/10.1038/s41598-017-06088-0
Zhang YY, Liu WN, Li YQ, Zhang XJ, Yang J, Luo XJ, Peng J (2019) Ligustroflavone reduces necroptosis in rat brain after ischemic stroke through targeting RIPK1/RIPK3/MLKL pathway. Naunyn Schmiedebergs Arch Pharmacol 392(9):1085–1095. https://doi.org/10.1007/s00210-019-01656-9
Tian J, Guo S, Chen H, Peng JJ, Jia MM, Li NS, Zhang XJ, Yang J, Luo XJ, Peng J (2018) Combination of emricasan with ponatinib synergistically reduces ischemia/reperfusion injury in rat brain through simultaneous Prevention of apoptosis and necroptosis. Transl Stroke Res 9(4):382–392. https://doi.org/10.1007/s12975-017-0581-z
Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN, Kannan HR, Menna AC, Voelkel NF, Abbate A (2011) The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci USA 108(49):19725–19730. https://doi.org/10.1073/pnas.1108586108
Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J, Hongo M, Noda T, Nakayama J, Sagara J, Taniguchi S, Ikeda U (2011) Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation 123(6):594–604. https://doi.org/10.1161/CIRCULATIONAHA.110.982777
Sandanger O, Ranheim T, Vinge LE, Bliksoen M, Alfsnes K, Finsen AV, Dahl CP, Askevold ET, Florholmen G, Christensen G, Fitzgerald KA, Lien E, Valen G, Espevik T, Aukrust P, Yndestad A (2013) The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovasc Res 99(1):164–174. https://doi.org/10.1093/cvr/cvt091
Nazir S, Gadi I, Al-Dabet MM, Elwakiel A, Kohli S, Ghosh S, Manoharan J, Ranjan S, Bock F, Braun-Dullaeus RC, Esmon CT, Huber TB, Camerer E, Dockendorff C, Griffin JH, Isermann B, Shahzad K (2017) Cytoprotective activated protein C averts Nlrp3 inflammasome-induced ischemia-reperfusion injury via mTORC1 inhibition. Blood 130(24):2664–2677. https://doi.org/10.1182/blood-2017-05-782102
Chen A, Chen Z, Xia Y, Lu D, Yang X, Sun A, Zou Y, Qian J, Ge J (2018) Liraglutide attenuates NLRP3 inflammasome-dependent pyroptosis via regulating SIRT1/NOX4/ROS pathway in H9c2 cells. Biochem Biophys Res Commun 499(2):267–272. https://doi.org/10.1016/j.bbrc.2018.03.142
Lei Q, Yi T, Chen C (2018) NF-kappaB-gasdermin D (GSDMD) axis couples oxidative stress and NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome-mediated cardiomyocyte pyroptosis following myocardial infarction. Med Sci Monit 24:6044–6052. https://doi.org/10.12659/MSM.908529
Ye B, Chen X, Dai S, Han J, Liang X, Lin S, Cai X, Huang Z, Huang W (2019) Emodin alleviates myocardial ischemia/reperfusion injury by inhibiting gasdermin D-mediated pyroptosis in cardiomyocytes. Drug Des Devel Ther 13:975–990. https://doi.org/10.2147/DDDT.S195412
Audia JP, Yang XM, Crockett ES, Housley N, Haq EU, O’Donnell K, Cohen MV, Downey JM, Alvarez DF (2018) Caspase-1 inhibition by VX-765 administered at reperfusion in P2Y12 receptor antagonist-treated rats provides long-term reduction in myocardial infarct size and preservation of ventricular function. Basic Res Cardiol 113(5):32. https://doi.org/10.1007/s00395-018-0692-z
Do Carmo H, Arjun S, Petrucci O, Yellon DM, Davidson SM (2018) The caspase 1 inhibitor VX-765 protects the isolated rat heart via the RISK pathway. Cardiovasc Drugs Ther 32(2):165–168. https://doi.org/10.1007/s10557-018-6781-2
Pan J, Han L, Guo J, Wang X, Liu D, Tian J, Zhang M, An F (2018) AIM2 accelerates the atherosclerotic plaque progressions in ApoE−/− mice. Biochem Biophys Res Commun 498(3):487–494. https://doi.org/10.1016/j.bbrc.2018.03.005
Zhang Y, Liu X, Bai X, Lin Y, Li Z, Fu J, Li M, Zhao T, Yang H, Xu R, Li J, Ju J, Cai B, Xu C, Yang B (2018) Melatonin prevents endothelial cell pyroptosis via regulation of long noncoding RNA MEG3/miR-223/NLRP3 axis. J Pineal Res. https://doi.org/10.1111/jpi.12449
Lin J, Shou X, Mao X, Dong J, Mohabeer N, Kushwaha KK, Wang L, Su Y, Fang H, Li D (2013) Oxidized low density lipoprotein induced caspase-1 mediated pyroptotic cell death in macrophages: implication in lesion instability? PLoS ONE 8(4):e62148. https://doi.org/10.1371/journal.pone.0062148
Li P, Zhong X, Li J, Liu H, Ma X, He R, Zhao Y (2018) MicroRNA-30c-5p inhibits NLRP3 inflammasome-mediated endothelial cell pyroptosis through FOXO3 down-regulation in atherosclerosis. Biochem Biophys Res Commun 503(4):2833–2840. https://doi.org/10.1016/j.bbrc.2018.08.049
Zhaolin Z, Jiaojiao C, Peng W, Yami L, Tingting Z, Jun T, Shiyuan W, Jinyan X, Dangheng W, Zhisheng J, Zuo W (2019) OxLDL induces vascular endothelial cell pyroptosis through miR-125a-5p/TET2 pathway. J Cell Physiol 234(5):7475–7491. https://doi.org/10.1002/jcp.27509
Wu X, Zhang H, Qi W, Zhang Y, Li J, Li Z, Lin Y, Bai X, Liu X, Chen X, Yang H, Xu C, Zhang Y, Yang B (2018) Nicotine promotes atherosclerosis via ROS-NLRP3-mediated endothelial cell pyroptosis. Cell Death Dis 9(2):171. https://doi.org/10.1038/s41419-017-0257-3
Zhang Y, Li X, Pitzer AL, Chen Y, Wang L, Li PL (2015) Coronary endothelial dysfunction induced by nucleotide oligomerization domain-like receptor protein with pyrin domain containing 3 inflammasome activation during hypercholesterolemia: beyond inflammation. Antioxid Redox Signal 22(13):1084–1096. https://doi.org/10.1089/ars.2014.5978
Lopez-Pastrana J, Ferrer LM, Li YF, Xiong X, Xi H, Cueto R, Nelson J, Sha X, Li X, Cannella AL, Imoukhuede PI, Qin X, Choi ET, Wang H, Yang XF (2015) Inhibition of caspase-1 activation in endothelial cells improves angiogenesis: a novel therapeutic potential for ischemia. J Biol Chem 290(28):17485–17494. https://doi.org/10.1074/jbc.M115.641191
Asbun J, Villarreal FJ (2006) The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J Am Coll Cardiol 47(4):693–700. https://doi.org/10.1016/j.jacc.2005.09.050
Boudina S, Abel ED (2010) Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord 11(1):31–39. https://doi.org/10.1007/s11154-010-9131-7
Litwin SE (2013) Diabetes and the heart: is there objective evidence of a human diabetic cardiomyopathy? Diabetes 62(10):3329–3330. https://doi.org/10.2337/db13-0683
Lee HM, Kim JJ, Kim HJ, Shong M, Ku BJ, Jo EK (2013) Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes 62(1):194–204. https://doi.org/10.2337/db12-0420
Yang F, Qin Y, Wang Y, Meng S, Xian H, Che H, Lv J, Li Y, Yu Y, Bai Y, Wang L (2019) Metformin inhibits the NLRP3 inflammasome via AMPK/mTOR-dependent effects in diabetic cardiomyopathy. Int J Biol Sci 15(5):1010–1019. https://doi.org/10.7150/ijbs.29680
Luo B, Li B, Wang W, Liu X, Xia Y, Zhang C, Zhang M, Zhang Y, An F (2014) NLRP3 gene silencing ameliorates diabetic cardiomyopathy in a type 2 diabetes rat model. PLoS ONE 9(8):e104771. https://doi.org/10.1371/journal.pone.0104771
Wang X, Pan J, Liu H, Zhang M, Liu D, Lu L, Tian J, Liu M, Jin T, An F (2019) AIM2 gene silencing attenuates diabetic cardiomyopathy in type 2 diabetic rat model. Life Sci 221:249–258. https://doi.org/10.1016/j.lfs.2019.02.035
Li X, Du N, Zhang Q, Li J, Chen X, Liu X, Hu Y, Qin W, Shen N, Xu C, Fang Z, Wei Y, Wang R, Du Z, Zhang Y, Lu Y (2014) MicroRNA-30d regulates cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic cardiomyopathy. Cell Death Dis 5:e1479. https://doi.org/10.1038/cddis.2014.430
Jeyabal P, Thandavarayan RA, Joladarashi D, Suresh Babu S, Krishnamurthy S, Bhimaraj A, Youker KA, Kishore R, Krishnamurthy P (2016) MicroRNA-9 inhibits hyperglycemia-induced pyroptosis in human ventricular cardiomyocytes by targeting ELAVL1. Biochem Biophys Res Commun 471(4):423–429. https://doi.org/10.1016/j.bbrc.2016.02.065
Yang F, Qin Y, Lv J, Wang Y, Che H, Chen X, Jiang Y, Li A, Sun X, Yue E, Ren L, Li Y, Bai Y, Wang L (2018) Silencing long non-coding RNA Kcnq1ot1 alleviates pyroptosis and fibrosis in diabetic cardiomyopathy. Cell Death Dis 9(10):1000. https://doi.org/10.1038/s41419-018-1029-4
Yang F, Wang Z, Wei X, Han H, Meng X, Zhang Y, Shi W, Li F, Xin T, Pang Q, Yi F (2014) NLRP3 deficiency ameliorates neurovascular damage in experimental ischemic stroke. J Cereb Blood Flow Metab 34(4):660–667. https://doi.org/10.1038/jcbfm.2013.242
Zhang D, Qian J, Zhang P, Li H, Shen H, Li X, Chen G (2019) Gasdermin D serves as a key executioner of pyroptosis in experimental cerebral ischemia and reperfusion model both in vivo and in vitro. J Neurosci Res 97(6):645–660. https://doi.org/10.1002/jnr.24385
Ito M, Shichita T, Okada M, Komine R, Noguchi Y, Yoshimura A, Morita R (2015) Bruton’s tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury. Nat Commun 6:7360. https://doi.org/10.1038/ncomms8360
She Y, Shao L, Zhang Y, Hao Y, Cai Y, Cheng Z, Deng C, Liu X (2019) Neuroprotective effect of glycosides in Buyang Huanwu Decoction on pyroptosis following cerebral ischemia-reperfusion injury in rats. J Ethnopharmacol 242:112051. https://doi.org/10.1016/j.jep.2019.112051
An P, Xie J, Qiu S, Liu Y, Wang J, Xiu X, Li L, Tang M (2019) Hispidulin exhibits neuroprotective activities against cerebral ischemia reperfusion injury through suppressing NLRP3-mediated pyroptosis. Life Sci 232:116599. https://doi.org/10.1016/j.lfs.2019.116599
Zhu S, Zhang Z, Jia LQ, Zhan KX, Wang LJ, Song N, Liu Y, Cheng YY, Yang YJ, Guan L, Min DY, Yang GL (2019) Valproic acid attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-pyroptosis pathways. Neurochem Int 124:141–151. https://doi.org/10.1016/j.neuint.2019.01.003
Bulluck H, Rosmini S, Abdel-Gadir A, White SK, Bhuva AN, Treibel TA, Fontana M, Ramlall M, Hamarneh A, Sirker A, Herrey AS, Manisty C, Yellon DM, Kellman P, Moon JC, Hausenloy DJ (2016) Residual myocardial iron following intramyocardial hemorrhage during the convalescent phase of reperfused ST-segment-elevation myocardial infarction and adverse left ventricular remodeling. Circ Cardiovasc Imaging. https://doi.org/10.1161/circimaging.116.004940
Baba Y, Higa JK, Shimada BK, Horiuchi KM, Suhara T, Kobayashi M, Woo JD, Aoyagi H, Marh KS, Kitaoka H, Matsui T (2018) Protective effects of the mechanistic target of rapamycin against excess iron and ferroptosis in cardiomyocytes. Am J Physiol Heart Circ Physiol 314(3):H659–H668. https://doi.org/10.1152/ajpheart.00452.2017
Fang X, Wang H, Han D, Xie E, Yang X, Wei J, Gu S, Gao F, Zhu N, Yin X, Cheng Q, Zhang P, Dai W, Chen J, Yang F, Yang HT, Linkermann A, Gu W, Min J, Wang F (2019) Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci USA 116(7):2672–2680. https://doi.org/10.1073/pnas.1821022116
Liu B, Zhao C, Li H, Chen X, Ding Y, Xu S (2018) Puerarin protects against heart failure induced by pressure overload through mitigation of ferroptosis. Biochem Biophys Res Commun 497(1):233–240. https://doi.org/10.1016/j.bbrc.2018.02.061
Gao M, Monian P, Quadri N, Ramasamy R, Jiang X (2015) Glutaminolysis and transferrin regulate ferroptosis. Mol Cell 59(2):298–308. https://doi.org/10.1016/j.molcel.2015.06.011
Garcia-Yebenes I, Sobrado M, Moraga A, Zarruk JG, Romera VG, Pradillo JM, Perez de la Ossa N, Moro MA, Davalos A, Lizasoain I (2012) Iron overload, measured as serum ferritin, increases brain damage induced by focal ischemia and early reperfusion. Neurochem Int 61(8):1364–1369. https://doi.org/10.1016/j.neuint.2012.09.014
Tuo QZ, Lei P, Jackman KA, Li XL, Xiong H, Li XL, Liuyang ZY, Roisman L, Zhang ST, Ayton S, Wang Q, Crouch PJ, Ganio K, Wang XC, Pei L, Adlard PA, Lu YM, Cappai R, Wang JZ, Liu R, Bush AI (2017) Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol Psychiatry 22(11):1520–1530. https://doi.org/10.1038/mp.2017.171
Guan X, Li X, Yang X, Yan J, Shi P, Ba L, Cao Y, Wang P (2019) The neuroprotective effects of carvacrol on ischemia/reperfusion-induced hippocampal neuronal impairment by ferroptosis mitigation. Life Sci 235:116795. https://doi.org/10.1016/j.lfs.2019.116795
Zille M, Karuppagounder SS, Chen Y, Gough PJ, Bertin J, Finger J, Milner TA, Jonas EA, Ratan RR (2017) Neuronal death after hemorrhagic stroke in vitro and in vivo shares features of ferroptosis and necroptosis. Stroke 48(4):1033–1043. https://doi.org/10.1161/STROKEAHA.116.015609
Li Q, Han X, Lan X, Gao Y, Wan J, Durham F, Cheng T, Yang J, Wang Z, Jiang C, Ying M, Koehler RC, Stockwell BR, Wang J (2017) Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight 2(7):e90777. https://doi.org/10.1172/jci.insight.90777
Pieper AA, Walles T, Wei G, Clements EE, Verma A, Snyder SH, Zweier JL (2000) Myocardial postischemic injury is reduced by polyADPripose polymerase-1 gene disruption. Mol Med 6(4):271–282
Liaudet L, Szabo E, Timashpolsky L, Virag L, Cziraki A, Szabo C (2001) Suppression of poly (ADP-ribose) polymerase activation by 3-aminobenzamide in a rat model of myocardial infarction: long-term morphological and functional consequences. Br J Pharmacol 133(8):1424–1430. https://doi.org/10.1038/sj.bjp.0704185
Yang Z, Zingarelli B, Szabo C (2000) Effect of genetic disruption of poly (ADP-ribose) synthetase on delayed production of inflammatory mediators and delayed necrosis during myocardial ischemia-reperfusion injury. Shock 13(1):60–66. https://doi.org/10.1097/00024382-200013010-00011
Xiao CY, Chen M, Zsengeller Z, Li H, Kiss L, Kollai M, Szabo C (2005) Poly(ADP-Ribose) polymerase promotes cardiac remodeling, contractile failure, and translocation of apoptosis-inducing factor in a murine experimental model of aortic banding and heart failure. J Pharmacol Exp Ther 312(3):891–898. https://doi.org/10.1124/jpet.104.077164
Pillai JB, Russell HM, Raman J, Jeevanandam V, Gupta MP (2005) Increased expression of poly(ADP-ribose) polymerase-1 contributes to caspase-independent myocyte cell death during heart failure. Am J Physiol Heart Circ Physiol 288(2):H486–H496. https://doi.org/10.1152/ajpheart.00437.2004
Andrabi SA, Kim NS, Yu SW, Wang H, Koh DW, Sasaki M, Klaus JA, Otsuka T, Zhang Z, Koehler RC, Hurn PD, Poirier GG, Dawson VL, Dawson TM (2006) Poly(ADP-ribose) (PAR) polymer is a death signal. Proc Natl Acad Sci USA 103(48):18308–18313. https://doi.org/10.1073/pnas.0606526103
Mashimo M, Kato J, Moss J (2013) ADP-ribosyl-acceptor hydrolase 3 regulates poly (ADP-ribose) degradation and cell death during oxidative stress. Proc Natl Acad Sci USA 110(47):18964–18969. https://doi.org/10.1073/pnas.1312783110
Mashimo M, Bu X, Aoyama K, Kato J, Ishiwata-Endo H, Stevens LA, Kasamatsu A, Wolfe LA, Toro C, Adams D, Markello T, Gahl WA, Moss J (2019) PARP1 inhibition alleviates injury in ARH3-deficient mice and human cells. JCI Insight. https://doi.org/10.1172/jci.insight.124519
Jin W, Xu W, Chen J, Zhang X, Shi L, Ren C (2016) Remote limb preconditioning protects against ischemia-induced neuronal death through ameliorating neuronal oxidative DNA damage and parthanatos. J Neurol Sci 366:8–17. https://doi.org/10.1016/j.jns.2016.04.038
Zhong H, Song R, Pang Q, Liu Y, Zhuang J, Chen Y, Hu J, Hu J, Liu Y, Liu Z, Tang J (2018) Propofol inhibits parthanatos via ROS-ER-calcium-mitochondria signal pathway in vivo and vitro. Cell Death Dis 9(10):932. https://doi.org/10.1038/s41419-018-0996-9
Wang K, An T, Zhou LY, Liu CY, Zhang XJ, Feng C, Li PF (2015) E2F1-regulated miR-30b suppresses Cyclophilin D and protects heart from ischemia/reperfusion injury and necrotic cell death. Cell Death Differ 22(5):743–754. https://doi.org/10.1038/cdd.2014.165
Xu T, Ding W, Ao X, Chu X, Wan Q, Wang Y, Xiao D, Yu W, Li M, Yu F, Wang J (2019) ARC regulates programmed necrosis and myocardial ischemia/reperfusion injury through the inhibition of mPTP opening. Redox Biol 20:414–426. https://doi.org/10.1016/j.redox.2018.10.023
Bibli SI, Papapetropoulos A, Iliodromitis EK, Daiber A, Randriamboavonjy V, Steven S, Brouckaert P, Chatzianastasiou A, Kypreos KE, Hausenloy DJ, Fleming I, Andreadou I (2019) Nitroglycerine limits infarct size through S-nitrosation of cyclophilin D: a novel mechanism for an old drug. Cardiovasc Res 115(3):625–636. https://doi.org/10.1093/cvr/cvy222
Nicolli A, Basso E, Petronilli V, Wenger RM, Bernardi P (1996) Interactions of cyclophilin with the mitochondrial inner membrane and regulation of the permeability transition pore, and cyclosporin A-sensitive channel. J Biol Chem 271(4):2185–2192. https://doi.org/10.1074/jbc.271.4.2185
Khaspekov L, Friberg H, Halestrap A, Viktorov I, Wieloch T (1999) Cyclosporin A and its nonimmunosuppressive analogue N-Me-Val-4-cyclosporin A mitigate glucose/oxygen deprivation-induced damage to rat cultured hippocampal neurons. Eur J Neurosci 11(9):3194–3198. https://doi.org/10.1046/j.1460-9568.1999.00743.x
Matsumoto S, Friberg H, Ferrand-Drake M, Wieloch T (1999) Blockade of the mitochondrial permeability transition pore diminishes infarct size in the rat after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab 19(7):736–741. https://doi.org/10.1097/00004647-199907000-00002
Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM (2012) p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell 149(7):1536–1548. https://doi.org/10.1016/j.cell.2012.05.014
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81872873 to Jun Peng, No. 81573430 to Xiu-Ju Luo). We sincerely thank Dr. Qingjie Li (Department of Internal Medicine, The University of Texas Medical Branch, USA) for his kind assistance in fixing the language problems in our manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lu, LQ., Tian, J., Luo, XJ. et al. Targeting the pathways of regulated necrosis: a potential strategy for alleviation of cardio-cerebrovascular injury. Cell. Mol. Life Sci. 78, 63–78 (2021). https://doi.org/10.1007/s00018-020-03587-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-020-03587-8