Abstract
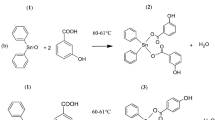
A series of asymmetric bis-1,2,4-triazoles (4a–l) were synthesized from respective 1,2,4-triazole-3-thiocarbohydrazides (2a, b) via base catalyzed dehydrative cyclization of thiosemicarbazide intermediates (3a–l). The synthesized compounds were characterized by IR, 1H-NMR, 13C-NMR, and Mass spectral studies. The asymmetric bis-1,2,4-triazole derivatives (4a–l) were evaluated for in vitro antioxidant activity by DPPH radical scavenging assay method. The compounds with significant antioxidant potential were evaluated for in vitro cytotoxicity by MTT assay method against HT29 (Human adenocarcinoma) and MDA-231 (Human breast cancer) cancer cell lines. All the synthesized compounds were evaluated for in vitro antibacterial activity against Bacillus subtilus (ATCC 6633), Staphylococcus aureus (ATCC-25923), Escherichia coli (ATCC-25922), and Pseudomonas aeruginosa (ATCC-27853).

Similar content being viewed by others
References
Al-Soud YA, Al-Masoudi NA (2004) DNA directed alkylating agents: synthesis, antitumor activity of DNA affinity of bis N,N′ trisubstituted 1,2,4-triazolo-piperazins. Il Farm 59:41–46. doi:10.1016/j.farmac.2003.08.006
Badran MM, Abouzid KAM, Hussein MHM (2003) Synthesis of certain substituted quinoxalines as antimicrobial agents (part II). Arch Pharm Res 26:107–113
Dabholkar VV, Ansari FY (2008) Synthesis and biological studies of bis (thiadiazole/triazole) by sonication. Acta Pol Pharm Drug Res 65:521–526
Demirbas N, Karaoglu SA, Demirbas A, Sancak K (2004) Synthesis and antimicrobial activities of some new 1-(5-phenylamino-[1,3,4]thiadiazol-2-yl)methyl-5-oxo-[1,2,4]triazole and 1-(4-phenyl-5-thioxo-[1,2,4]triazol-3-yl)methyl-5-oxo-[1,2,4] triazole derivatives. Eur J Med Chem 39:793–804
Denny WA (2003) Synthetic DNA targeted chemotherapeutic agents and related tumor activated prodrugs. In: Abhram DJ (ed) Bergers medicinal chemistry and drug discovery, vol 5, 6th edn. Wiley, Hoboken, pp 74–75
Qu F, Hang JH, Du J, Newton MG, Chu CK (1999) Asymmetric Synthesis of (2′R,4′R) and (2′S,4′s)- 1,3-dioxolanyl triazole C-nucleosides as potential antiviral and anticancer agents. Tetrahedron 55:9073–9088
Ghorab MM, El-Sharief AMS, Ammar YA, Mohamed SI (2000) Synthesis and radiation stability of novel biologically active sulfur compounds derived from 1,2-bis(4-amino-5-mercapto-s-triazol-3-yl)ethane. Il Farm 55:354–361
Holla BS, Gonsalves R, Shenoy S (2000) Synthesis and anti bacterial studies of a new series of 1,2-bis(1,3,4-oxadiazol-2-yl)ethanes and 1,2-bis(4-amino-1,2,4 triazolo-3-yl)ethanes. Eur J Med Chem 35:267–271
Holla BS, Sarojini BK, Rao BS, Akberali PM, Suchetha Kumari N, Shetty V (2001) Synthesized some halogen containing 1,2,4-triazolo-1,3,4-thiadiazines and performed their anticancer screening: II. Farmaco 56:565–570
Holla BS, Poojary KN, Rao BS, Shivananda MK (2002) New bis-amino mercapto triazoles and bis triazolo thiadiazoles as possible anticancer agents. Eur J Med Chem 37:511–517
Hugo WB, Russell AD (1997) Pharmaceutical microbiology, 1st edn. Blackwell, Oxford, London, p. 663
Manjula SN, Noolvi NM, Parihar KV, Manohara Reddy SA, Ramani J, Gadad AK, Singh G, Gopalan Kutty N, Mallikarjuna Rao C (2009) Synthesis and antitumor activity of optically active thiourea and their 2-amino benzothiazole derivatives: a novel class of anti cancer agents. Eur J Med Chem 44:2923–2929. doi:10.1016/j.ejmech.2008.12.002
Molinari A, Ojeda C, Oliva A, Miguel del Corral JM, Castro MA, Cuevas C, Feliciano AS (2009) Synthesis and cytotoxic evaluation of 6-(3-pyrazolylpropyl) derivatives of 1,4-naphthohydroquinone-1,4-diacetate. Arch Pharm Chem Life Sci 342:591–599. doi:10.1002/ardp.200900041
Omar MT (1997) Synthesis of new xanthenone derivatives of expected antibilharzial activity. Arch Pharm Res 20:602–609
Pujar GV, Manohar KV, Udupi RH, Purohit MN, Chandrasekar MJN (2006) Synthesis and antimicrobial activity of 4-substituted-5-mercapto 3-carboxamido triazoles. Indian J Heterocycl Chem 16(3):69–70
Purohit M, Mayur YC (2012) Synthesis, in vitro cytotoxicity and anti-microbialstudies of 1,4-bis(4-substituted-5-mercapto-1,2,4-triazol-3-1)butanes. Med Chem Res 21(2):174–184. doi:10.1007/s00044-010-9517-9
Purohit M, Prasad VVSR, Mayur YC (2011) Synthesis and cytotoxicity of bis-1,3,4-oxadiazoles and bis-pyrazoles derived from 1,4-bis[5-thio-4-substituted-1,2,4-triazol-3-yl]-butane and their DNA binding. Arch Pharm Chem Life Sci 11:248–254. doi:10.1002/ardp.201000177
Roopan SM, Maiyalagan T, Khan FN (2008) Solvent free synthesis of some quinazoli-4(3H)-ones derivatives. Can J Chem 86:1019–1025
Rostom SAF, Ashour HMA, El Razik HAA (2009) Synthesis and biological evaluation of some novel poly substituted pyrimidine derivatives as potential anti microbial and anti cancer agents. Arch Pharm Chem Life Sci 342:299–310. doi:10.1002/ardp.200800223
Spicer JA, Gamage SA, Rewcastle GW, Finlay GJ, Bridewell DJA, Baguley BC, Denny WA (2000) Bis (phenazine 1-carboxamide): structure activity relationships for a new class of dual topoisomerase I/II directed anti cancer drugs. J Med Chem 43:1350–1358. doi:10.1021/jm990423f
Sztanke K, Tuzimski T, Rzymowska J, Pasternak K, Szerszen MK (2008) Synthesized and determined the lipophilicity, anticancer and antimicrobial properties of some fused 1,2,4-triazole derivatives. Eur J Med Chem 43:404–419
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer statistics. CA Cancer J Clin 59:225–249
Yong P, Yang Q, Quin X (2005) Novel DNA bis-intercalators of isoquiolino-[4,5-bc]-acridones: design, synthesis and evaluation of cytotoxic activity. Tetrahedron 61:11895–11901. doi:10.1016/j.tet.2005.09.065
Yuksek H, Kolayli S, Kucuk M, Yuksek MO, Ocak U, Sahinbas E, Sivirikaya E, Ocak M (2006). Synthesis and antioxidant activities of some 4-benzylidenamino-4,5-dihydro-1H-1,2,4-triazol-5-one derivatives. Indian J. Chem 45(B):715–718
Zhang DC, Guo L, Li ZS, Wang HL, Ye CY (2006) Carboxyamido-triazole inhibits proliferation of human breast cancer cells via G2/M cell cycle arrest and apoptosis. Bioorg Med Chem Lett 15:5407–5411
Acknowledgments
Authors are thankful to The Principal, JSS College of Pharmacy, Mysore, India for providing necessary facilities. Authors express thanks to The Director, NMR research centre, Indian Institute of Science, Bangalore for spectral data.
Conflict of interest
Authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, R., Pujar, G.V., Purohit, M.N. et al. Synthesis, in vitro cytotoxicity, and antibacterial studies of new asymmetric bis-1,2,4-triazoles. Med Chem Res 22, 2163–2173 (2013). https://doi.org/10.1007/s00044-012-0209-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-012-0209-5