Abstract
Purpose
Bone regeneration is an important concern in periodontal treatment and implant dentistry. Different biomaterials and surgical techniques have been used for this purpose. The aim of the present study was to compare the effect of nanocrystalline hydroxyapatite and human freeze-dried bone graft (FDBG) in regeneration of rabbit calvarium bony defects by histologic and histomorphometric evaluation.
Methods
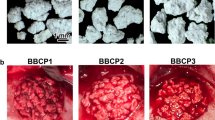
In this experimental study, three similar defects, measuring 8 mm in diameter, were created in the calvaria of 16 white New Zealand rabbits. Two defects were filled with FDBG and nanocrystalline hydroxyapatite silica gel, while the other one remained unfilled to be considered as control. All the defects were covered with collagen membranes. During the healing period, two animals perished; so 14 rabbits were divided into two groups: half of them were euthanized after 6 weeks of healing and the other half after 12 weeks. The specimens were subjected to histologic and histomorphometric examinations for assessment of the following variables: percentage of bone formation and residual graft material, inflammation scores, patterns of bone formation and type of newly formed bone.
Results
The percentages of new bone formation after 6 weeks were 14.22 ± 7.85, 21.57 ± 6.91, and 20.54 ± 10.07% in FDBG, NanoBone, and control defects. These values were 27.54 ± 20.19, 23.86 ± 6.27, and 26.48 ± 14.18% in 12-week specimens, respectively. No significant differences were found in the amount of bone formation between the groups. With regard to inflammation, the control and NanoBone groups showed significantly less inflammation compared to FDBG at the 6-week healing phase (P = 0.04); this difference was not significant in the 12-week specimens.
Conclusions
Based on the results of this experimental study, both NanoBone and FDBG exhibited a similar effect on bone formation.
Zusammenfassung
Ziel
Die Regeneration neuen Knochens ist ein wichtiges Ziel im Rahmen der Parodontologie und der Implantologie. In diesem Kontext sind unterschiedliche Biomaterialen und chirurgische Verfahren eingesetzt worden. Ziel der vorgestellten Studie war es, am Modell von Schädelkalottendefekten des Kaninchens die Effekte von nanokristallinem Hydroxylapatit und gefriergetrocknetem humanem Knochen (“freeze-dried bone graft”, FDBG) bei der Regeneration histologisch und histomorphometrisch zu evaluieren.
Methoden
In dieser experimentellen Studie wurden je 3 ähnliche Defekte (Durchmesser 8 mm) in der Kalotte von 16 weißen Neuseelandkaninchen erzeugt. Zwei wurden mit FDBG und nanokristallinem Hydroxylapatitgel aufgefüllt, der dritte blieb unbehandelt und diente als Kontrolle. Alle Defekte wurden mit Kollagenmembranen abgedeckt. Im Verlauf der Heilungsperiode verstarben 2 Tiere; die übrigen 14 wurden in 2 Gruppen aufgeteilt: eine Gruppe wurde nach 6, die andere nach 12 Wochen getötet. Die Biopsien wurden histologisch und histomorphometrisch untersucht im Hinblick auf folgende Variablen: der Prozentsatz neu gebildeten Knochens und des verbliebenen Biomaterials, Inflammation/Entzündung, Muster und Struktur der Knochenneubildung und Art des neu gebildeten Knochens.
Results
Die Prozentsätze neu gebildeten Knochens nach 6 Wochen lagen bei 14,22 ± 7,85, 21,57 ± 6,91 und 20,54 ± 10,07% bei FDBG, NanoBone bzw. Kontrollen. In den 12-Wochen-Proben lagen die entsprechenden Werte bei 27,54 ± 20,19, 23,86 ± 6,27 und 26,48 ± 14,18%. Hinsichtlich der Menge des neu gebildeten Knochens ließen sich keine signifikanten Unterschiede nachweisen. Nach 6 Wochen war die Inflammation/Entzündung in der Kontroll- und in der NanoBone-Gruppe deutlich (p = 0,04) weniger ausgeprägt als in der FDBG-Gruppe, in den 12-Wochen-Proben war dieser Unterschied nicht mehr signifikant.
Schlussfolgerungen
Auf der Basis der in der vorgestellten Studie erreichten Ergebnisse zeigten sowohl NanoBone als auch FDBG ähnliche Effekte hinsichtlich der Knochenbildung.


Similar content being viewed by others
References
Behairy Y, Jasty M (1999) Bone grafts and bone substitutes in hip and knee surgery. Orthop Clin North Am 30:661–671
Behfarnia P, Shahabooei M, Mashhadiabbas F, Fakhari E (2012) Comparison of bone regeneration using three demineralized freeze-dried bone allografts: a histological and histomorphometric study in rabbit calvaria. Dent Res J (Isfahan) 9:554–560
Behnia H, Khojasteh A, Kiani MT, Khoshzaban A, Mashhadi Abbas F, Bashtar M et al (2013) Bone regeneration with a combination of nanocrystalline hydroxyapatite silica gel, platelet-rich growth factor, and mesenchymal stem cells: a histologic study in rabbit calvaria. Oral Surg Oral Med Oral Pathol Oral Radiol 115:e7–e15
Behnia H, Khoshzaban A, Zarinfar M, Mashhadi Abbas F, Bahraminasab H, Khojasteh A (2012) Histological Evaluation of regeneration in rabbit calvarial bone defects using demineralized bone matrix, mesenchymal stem cells and platelet rich in growth factors. J Dent Sch (Journal of Dental School, Shahid Beheshti University of Medical Sciences) 30:143–154
Boden SD, Schimandle JH, Hutton WC (1995) An experimental lumbar intertransverseprocess spinal fusion model. Radiographic, histologic, and biomechanical healing characteristics. Spine 20:412–420
Borie E, Fuentes R, Del Sol M, Oporto G, Engelke W (2011) The influence of FDBA and autogenous bone particles on regeneration of calvaria defects in the rabbit: a pilot study. Ann Anat 193:412–417
Bos GD, Goldberg VM, Powell AE, Heiple KG, Zika JM (1983) The effect of histocompatibility matching on canine frozen bone allografts. J Bone Joint Surg Am 65:89–96
Cammack GV 2nd, Nevins M, Clem DS 3rd, Hatch JP, Mellonig JT (2005) Histologic evaluation of mineralized and demineralized freeze-dried bone allograft for ridge and sinus augmentations. Int J Periodontics Restorative Dent 25:231–237
Cornell CN (2004) Osteobiologics. Bull Hosp Jt Dis 62:13–17
Darby I, Chen S, Buser D (2009) Ridge preservation techniques for implant therapy. Int J Oral Maxillofac Implants 24(suppl):260–271
Fucini S, Quintero G, Gher M, Black B, Richardson A (1993) Small versus large particles of demineralized freeze-dried bone allografts in human intrabony periodontal defects. J Periodontol 64:844–847
Gerber T, Holzhüter G, Götz W, Bienengräber V, Henkel K, Rumpel E (2006) Nanostructuring of Biomaterials—a pathway to bone grafting substitute. Eur J Trauma 32:132–140
Gerike W, Bienengräber V, Henkel KO, Bayerlein T, Proff P, Gedrange T et al (2006) The manufacture of synthetic non-sintered and degradable bone grafting substitutes. Folia Morphol (Warsz) 65:54–55
Goldberg VM, Stevenson S (1987) Natural history of autografts and allografts. Clin Orthop Relat Res 225:7–16
Greenwald JA, Mehrara BJ, Spector JA, Chin GS, Steinbrech DS, Saadeh PB et al (2000) Biomolecular mechanisms of calvarial bone induction: immature versus mature dura mater. Plast Reconstr Surg 105:1382–1392
Haddad AJ, Peel SA, Clokie CM, Sándor GK (2006) Closure of rabbit calvarial critical-sized defects using protective composite allogeneic and alloplastic bone substitutes. J CraniofacSurg 17:926–934
Harms C, Helms K, Taschner T, Stratos I, Ignatius A, Gerber T et al (2012) Osteogenic capacity of nano-crystalline bone cement in a weight-bearing defect at the ovine tibial metaphysis. Int J NanoMedicine 7:2883–2889
Henkel K-O, Gerber T, Lenz S, Gundlach KKH (2006) Macroscopical, histological, and morphometric studies of porous bone-replacement materials in minipigs 8 months after implantation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 102:606–613
Holmes RE, Bucholz RW, Mooney V (1986) Porous hydroxyapatite as a bone-graft substitute in metaphyseal defects. A histometric study. J Bone Joint Surg Am 68:904–911
Iasella J, Greenwell H, Miller R (2003) Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: a clinical and histologic study in humans. J Periodontol 74:990–999
Jan AM, Sándor GK, Iera D, Mhawi A, Peel S, Evans AW et al (2006) Hyperbaric oxygen results in an increase in rabbit calvarial critical sized defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101:144–149
Jarcho M (1981) Calcium phosphate ceramics as hard tissue prosthetics. ClinOrthopRelat Res 157:259–278
Kramer IR, Killey HC, Wright HC (1968) A histological and radiological comparison of the healing of defects of the rabbit calvarium with and without implanted heterogeneous anorganic bone. Arch Oral Biol 13:1095–1106
Kruse A, Jung RE, Nicholls F, Zwahlen RA, Hämmerle CH, Weber FE (2011) Bone regeneration in the presence of a synthetic hydroxyapatite/silica oxide-based and a xenogenic hydroxyapatite-based bone substitute material. Clin Oral Implants Res 22:506–511
Lee DW, Koo KT, Seol YJ, Lee YM, Ku Y, Rhyu IC et al (2010) Bone regeneration effects of human allogenous bone substitutes: a preliminary study. J Periodontal Implant Sci 40:132–138
Liu Y, Lu Y, Tian X, Cui G, Zhao Y, Yang Q et al (2009) Segmental bone regeneration using an rhBMP-2-loaded gelatin/nanohydroxyapatite/fibrin scaffold in a rabbit model. Biomaterials 30:6276–6285
Ludwig SC, Boden SD (1999) Osteoinductive bone graft substitutes for spinal fusion: a basic science summary. Orthop Clin North Am 30:635–645
Misch CE, Misch-Dietsh F (2008) Key to bone grafting and bone grafting materials. In: Misch CE (ed) Contemporary implant dentistry, 3rd edn. Mosby Elsevier, Missouri, pp 839–869
Moghadam HG, Sandor GK, Holmes HH, Clokie MLC (2004) Histomorphometric evaluation of bone regeneration using allogeneic and alloplastic bone substitutes. J Oral Maxillofac Surg 62:202–213
Moronme M, Boden S (1998) Experimental posterolateral lumbar spinal fusion with a demineralized bone matrix gel. Spine 23:159–167
Pelegrine A, Aloise A, Zimmermann A, de Mello E, Oliveira R, Ferreira LM, Oliveira R et al (2014) Repair of critical-size bone defects using bone marrow stromal cells: a histomorphometric study in rabbit calvaria. Part I: use of fresh bone marrow or bone marrow mononuclear fraction. Clin Oral Implants Res 25:567–572
Piattelli A, Scarano A, Corigliano M, Piattelli M (1996) Comparison of bone regeneration with the use of mineralized and demineralized freeze-dried bone allografts: a histological and histochemical study in man. Biomaterials 17:1127–1131
Kao Richard T, Takei Henry H, Cochran David L, Nevins Marc L (2015) Periodontal Regeneration and Reconstructive Surgery. In: Newman MG, Takei HH, Klokkevold PR, Carranza FA (eds) Carranza’s Clinical Periodontology, 12th edn. Elsevier Saunders, St. Louis, pp 610–620
Rokn A, Moslemi N, Eslami B, Abadi HK, Paknejad M (2012) Histologic Evaluation of bone healing following application of anorganic bovine bone and β-tricalcium phosphate in rabbit calvaria. J Dent (Tehran) 9:35–40
Rokn AR, Khodadoostan MA, Reza Rasouli Ghahroudi AA, Motahhary P, Kharrazi Fard MJ, Bruyn HD et al (2011) Bone formation with two types of grafting materials: a histologic and histomorphometric study. Open Dent J. 5:96–104
Rummelhart JM, Mellonig JT, Gray JL, Towle HJ (1989) A comparison of freeze-dried bone allograft and demineralized freeze-dried bone allograft in human periodontal osseous defects. J Periodontol 60:655–663
Schmitz JP, Hollinger JO (1986) The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res 205:299–308
Shigeyama Y, D’Errico JA, Stone R, Somerman MJ (1995) Commercially-prepared allograft material has biological activity in vitro. J Periodontol 66:478–487
Sohn JY, Park JC, Um YJ, Jung UW, Kim CS, Cho KS et al (2010) Spontaneous healing capacity of rabbit cranial defects of various sizes. J Periodontal Implant Sci 40:180–187
Tay BK, Patel VV, Bradford DS (1999) Calcium sulfate- and calcium phosphate-based bone substitutes. Mimicry of the mineral phase of bone. Orthop Clin North Am 30:615–623
Torres 1, Tamimi FM, Tresguerres IF, Alkhraisat MH, Khraisat A, Lopez-Cabarcos E, et al. (2008) Effect of solely applied platelet-rich plasma on osseous regeneration compared to Bio-Oss: a morphometric and densitometric study on rabbit calvaria. Clin Implant Dent Relat Res 10:106–112
Wood RA, Mealey BL (2012) Histologic comparison of healing after tooth extraction with ridge preservation using mineralized versus demineralized freeze-dried bone allograft. J Periodontol 83:329–336
Younger EM, Chapman MW (1989) Morbidity at bone graft donor sites. J Orthop Trauma 3:192–195
Yukna RA, Vastardis S (2005) Comparative evaluation of decalcified and non-decalcified freeze-dried bone allografts in rhesus monkeys. I. Histologic findings. J Periodontol 76:57–65
Yun PY, Kim YK, Jeong KI, Park JC, Choi YJ (2014) Influence of bone morphogenetic protein and proportion of hydroxyapatite on new bone formation in biphasic calcium phosphate graft: two pilot studies in animal bony defect model. J Craniomaxillofac Surg 42:1909–1917
Zakaria SM, Sharif Zein SH, Othman MR, Yang F, Jansen J (2013) Nanophase hydroxyapatite as a biomaterial in advanced hard tissue engineering: a review. Tissue Eng Part B Rev 19:431–441
Acknowledgements
We would like to thank Dr M.J. Kharrazi Fard from Tehran University of Medical Sciences for statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R Sadeghi, M. Najafi, H. Semyari, and F. Mashhadiabbas declare that they have no conflict of interest.
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Rokhsareh Sadeghi: Assistant Professor; Mohammad Najafi: Assistant Professor; Hassan Semyari: Associate Professor; Fatemeh Mashhadiabbas: Associate Professor.
Rights and permissions
About this article
Cite this article
Sadeghi, R., Najafi, M., Semyari, H. et al. Histologic and histomorphometric evaluation of bone regeneration using nanocrystalline hydroxyapatite and human freeze-dried bone graft. J Orofac Orthop 78, 144–152 (2017). https://doi.org/10.1007/s00056-016-0067-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00056-016-0067-8