Abstract
Purpose
This study aimed to evaluate the application of apparent diffusion coefficient (ADC) histogram analysis to differentiate posterior fossa tumors (PFTs) in children.
Methods
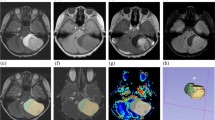
A total of 175 pediatric patients with PFT, including 75 pilocytic astrocytomas (PA), 59 medulloblastomas, 16 ependymomas, and 13 atypical teratoid rhabdoid tumors (ATRT), were analyzed. Tumors were visually assessed using DWI trace and conventional MRI images and manually segmented and post-processed using parametric software (pMRI). Furthermore, tumor ADC values were normalized to the thalamus and cerebellar cortex. The following histogram metrics were obtained: entropy, minimum, 10th, and 90th percentiles, maximum, mean, median, skewness, and kurtosis to distinguish the different types of tumors. Kruskal Wallis and Mann-Whitney U tests were used to evaluate the differences. Finally, receiver operating characteristic (ROC) curves were utilized to determine the optimal cut-off values for differentiating the various PFTs.
Results
Most ADC histogram metrics showed significant differences between PFTs (p < 0.001) except for entropy, skewness, and kurtosis. There were significant pairwise differences in ADC metrics for PA versus medulloblastoma, PA versus ependymoma, PA versus ATRT, medulloblastoma versus ependymoma, and ependymoma versus ATRT (all p < 0.05). Our results showed no significant differences between medulloblastoma and ATRT. Normalized ADC data showed similar results to the absolute ADC value analysis. ROC curve analysis for normalized ADCmedian values to thalamus showed 94.9% sensitivity (95% CI: 85–100%) and 93.3% specificity (95% CI: 87–100%) for differentiating medulloblastoma from ependymoma.
Conclusion
ADC histogram metrics can be applied to differentiate most types of posterior fossa tumors in children.



Similar content being viewed by others
References
Hanzlik E, Woodrome SE, Abdel-Baki M, Geller TJ, Elbabaa SK. A systematic review of neuropsychological outcomes following posterior fossa tumor surgery in children. Childs Nerv Syst. 2015;31:1869–75.
Poretti A, Meoded A, Huisman TA. Neuroimaging of pediatric posterior fossa tumors including review of the literature. J Magn Reson Imaging. 2012;35:32–47.
Prasad KSV, Ravi D, Pallikonda V, Raman BVS. Clinicopathological study of pediatric posterior fossa tumors. J Pediatr Neurosci. 2017;12:245–50.
Brandão LA, Young Poussaint T. Posterior fossa tumors. Neuroimaging Clin N Am. 2017;27:1–37.
Plaza MJ, Borja MJ, Altman N, Saigal G. Conventional and advanced MRI features of pediatric intracranial tumors: posterior fossa and suprasellar tumors. AJR Am J Roentgenol. 2013;200:1115–24.
Huisman TA. Posterior fossa tumors in children: differential diagnosis and advanced imaging techniques. Neuroradiol J. 2007;20:449–60.
Jaremko JL, Jans LBO, Coleman LT, Ditchfield MR. Value and limitations of diffusion-weighted imaging in grading and diagnosis of pediatric posterior fossa tumors. AJNR Am J Neuroradiol. 2010;31:1613–6.
Yamasaki F, Kurisu K, Satoh K, Arita K, Sugiyama K, Ohtaki M, Takaba J, Tominaga A, Hanaya R, Yoshioka H, Hama S, Ito Y, Kajiwara Y, Yahara K, Saito T, Thohar MA. Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology. 2005;235:985–91.
Just N. Improving tumour heterogeneity MRI assessment with histograms. Br J Cancer. 2014;111:2205–13.
Gihr GA, Horvath-Rizea D, Hekeler E, Ganslandt O, Henkes H, Hoffmann KT, Scherlach C, Schob S. Histogram Analysis of Diffusion Weighted Imaging in Low-Grade Gliomas: in vivo Characterization of Tumor Architecture and Corresponding Neuropathology. Front Oncol. 2020;10:206.
Reddy N, Ellison DW, Soares BP, Carson KA, Huisman TAGM, Patay Z. Pediatric posterior fossa medulloblastoma: the role of diffusion imaging in identifying molecular groups. J Neuroimaging. 2020;30:503–11.
De Jay N, Papillon-Cavanagh S, Olsen C, El-Hachem N, Bontempi G, Haibe-Kains B. mRMRe: an R package for parallelized mRMR ensemble feature selection. Bioinformatics. 2013;29:2365–8.
Chourmouzi D, Papadopoulou E, Konstantinidis M, Syrris V, Kouskouras K, Haritanti A, Karkavelas G, Drevelegas A. Manifestations of pilocytic astrocytoma: a pictorial review. Insights Imaging. 2014;5:387–402.
Koeller KK, Rushing EJ. From the archives of the AFIP: medulloblastoma: a comprehensive review with radiologic-pathologic correlation. Radiographics. 2003;23:1613–37.
Koob M, Girard N. Cerebral tumors: specific features in children. Diagn Interv Imaging. 2014;95:965–83.
Choudhri AF, Siddiqui A, Klimo P. Pediatric cerebellar tumors: emerging imaging techniques and advances in understanding of genetic features. Magn Reson Imaging Clin N Am. 2016;24:811–21.
Meyers SP, Kemp SS, Tarr RW. MR imaging features of medulloblastomas. AJR Am J Roentgenol. 1992;158:859–65.
Yuh EL, Barkovich AJ, Gupta N. Imaging of ependymomas: MRI and CT. Childs Nerv Syst. 2009;25:1203–13.
Panigrahy A, Krieger MD, Gonzalez-Gomez I, Liu X, McComb JG, Finlay JL, Nelson MD Jr, Gilles FH, Blüml S. Quantitative short echo time 1H-MR spectroscopy of untreated pediatric brain tumors: preoperative diagnosis and characterization. AJNR Am J Neuroradiol. 2006;27:560–72.
Wu G, Pang H, Ghimire P, Liu G. (1)H magnetic resonance spectroscopy and diffusion weighted imaging findings of medulloblastoma in 3.0T MRI: a retrospective analysis of 17 cases. Neural Regen Res. 2012;7:2554–9.
Arle JE, Morriss C, Wang ZJ, Zimmerman RA, Phillips PG, Sutton LN. Prediction of posterior fossa tumor type in children by means of magnetic resonance image properties, spectroscopy, and neural networks. J Neurosurg. 1997;86:755–61.
Schneider JF, Confort-Gouny S, Viola A, Le Fur Y, Viout P, Bennathan M, Chapon F, Figarella-Branger D, Cozzone P, Girard N. Multiparametric differentiation of posterior fossa tumors in children using diffusion-weighted imaging and short echo-time 1H-MR spectroscopy. J Magn Reson Imaging. 2007;26:1390–8.
Sutton LN, Wehrli SL, Gennarelli L, Wang Z, Zimmerman R, Bonner K, Rorke LB. High-resolution 1H-magnetic resonance spectroscopy of pediatric posterior fossa tumors in vitro. J Neurosurg. 1994;81:443–8.
Rueckriegel SM, Driever PH, Bruhn H. Supratentorial neurometabolic alterations in pediatric survivors of posterior fossa tumors. Int J Radiat Oncol Biol Phys. 2012;82:1135–41.
Dong J, Li L, Liang S, Zhao S, Zhang B, Meng Y, Zhang Y, Li S. Differentiation Between Ependymoma and Medulloblastoma in Children with Radiomics Approach. Acad Radiol. 2021;28:318–27.
Payabvash S, Tihan T, Cha S. Differentiation of cerebellar hemisphere tumors: combining apparent diffusion coefficient histogram analysis and structural MRI features. J Neuroimaging. 2018;28:656–65.
Zitouni S, Koc G, Doganay S, Saracoglu S, Gumus KZ, Ciraci S, Coskun A, Unal E, Per H, Kurtsoy A, Kontas O. Apparent diffusion coefficient in differentiation of pediatric posterior fossa tumors. Jpn J Radiol. 2017;35:448–53.
Wagner MW, Narayan AK, Bosemani T, Huisman TA, Poretti A. Histogram Analysis of Diffusion Tensor Imaging Parameters in Pediatric Cerebellar Tumors. J Neuroimaging. 2016;26:–5.
Poretti A, Meoded A, Cohen KJ, Grotzer MA, Boltshauser E, Huisman TA. Apparent diffusion coefficient of pediatric cerebellar tumors: a biomarker of tumor grade? Pediatr Blood Cancer. 2013;60:2036–41.
Rumboldt Z, Camacho DLA, Lake D, Welsh CT, Castillo M. Apparent diffusion coefficients for differentiation of cerebellar tumors in children. AJNR Am J Neuroradiol. 2006;27:1362–9.
Bidiwala S, Pittman T. Neural network classification of pediatric posterior fossa tumors using clinical and imaging data. Pediatr Neurosurg. 2004;40:8–15.
Wang Z, Sutton LN, Cnaan A, Haselgrove JC, Rorke LB, Zhao H, Bilaniuk LT, Zimmerman RA. Proton MR spectroscopy of pediatric cerebellar tumors. AJNR Am J Neuroradiol. 1995;16:1821–33.
Yeom KW, Mitchell LA, Lober RM, Barnes PD, Vogel H, Fisher PG, Edwards MS. Arterial spin-labeled perfusion of pediatric brain tumors. AJNR Am J Neuroradiol. 2014;35:395–401.
Kerleroux B, Cottier JP, Janot K, Listrat A, Sirinelli D, Morel B. Posterior fossa tumors in children: radiological tips & tricks in the age of genomic tumor classification and advance MR technology. J Neuroradiol. 2020;47:46–53.
Blüml S, Margol AS, Sposto R, Kennedy RJ, Robison NJ, Vali M, Hung LT, Muthugounder S, Finlay JL, Erdreich-Epstein A, Gilles FH, Judkins AR, Krieger MD, Dhall G, Nelson MD, Asgharzadeh S. Molecular subgroups of medulloblastoma identification using noninvasive magnetic resonance spectroscopy. Neuro Oncol. 2016;18:126–31.
Davies NP, Wilson M, Harris LM, Natarajan K, Lateef S, Macpherson L, Sgouros S, Grundy RG, Arvanitis TN, Peet AC. Identification and characterisation of childhood cerebellar tumours by in vivo proton MRS. NMR Biomed. 2008;21:908–18.
Moreno-Torres A, Martínez-Pérez I, Baquero M, Campistol J, Capdevila A, Arús C, Pujol J. Taurine detection by proton magnetic resonance spectroscopy in medulloblastoma: contribution to noninvasive differential diagnosis with cerebellar astrocytoma. Neurosurgery. 2004;55:824–9; discussion 829.
Schneider JF, Viola A, Confort-Gouny S, Ayunts K, Le Fur Y, Viout P, Bennathan M, Chapon F, Figarella-Branger D, Cozzone P, Girard N. Infratentorial pediatric brain tumors: the value of new imaging modalities. J Neuroradiol. 2007;34:49–58.
Gimi B, Cederberg K, Derinkuyu B, Gargan L, Koral KM, Bowers DC, Koral K. Utility of apparent diffusion coefficient ratios in distinguishing common pediatric cerebellar tumors. Acad Radiol. 2012;19:794–800.
Tuntiyatorn L, Nantawas B, Sirachainan N, Larbcharoensub N, Visudtibhan A, Hongeng S. Apparent diffusion coefficients in evaluation of pediatric brain tumors. J Med Assoc Thai. 2013;96:178–84.
Yamashita Y, Kumabe T, Higano S, Watanabe M, Tominaga T. Minimum apparent diffusion coefficient is significantly correlated with cellularity in medulloblastomas. Neurol Res. 2009;31:940–6.
Ji YM, Geng DY, Huang BC, Li YX, Ren G, Zhu L. Value of diffusion-weighted imaging in grading tumours localized in the fourth ventricle region by visual and quantitative assessments. J Int Med Res. 2011;39:912–9.
Pierce T, Kranz PG, Roth C, Leong D, Wei P, Provenzale JM. Use of apparent diffusion coefficient values for diagnosis of pediatric posterior fossa tumors. Neuroradiol J. 2014;27:233–44.
Koral K, Alford R, Choudhury N, Mossa-Basha M, Gargan L, Gimi B, Gao A, Zhang S, Bowers DC, Koral KM, Izbudak I. Applicability of apparent diffusion coefficient ratios in preoperative diagnosis of common pediatric cerebellar tumors across two institutions. Neuroradiology. 2014;56:781–8.
Koral K, Mathis D, Gimi B, Gargan L, Weprin B, Bowers DC, Margraf L. Common pediatric cerebellar tumors: correlation between cell densities and apparent diffusion coefficient metrics. Radiology. 2013;268:532–7.
Sathyakumar K, Mani S, Pathak GH, Prabhu K, Chacko AG, Chacko G. Neuroimaging of pediatric infratentorial tumors and the value of diffusion-weighted imaging (DWI) in determining tumor grade. Acta Radiol. 2021;62:533–40.
Phuttharak W, Wannasarnmetha M, Wara-Asawapati S, Yuthawong S. Diffusion MRI in evaluation of pediatric posterior fossa tumors. Asian Pac J Cancer Prev. 2021;22:1129–36.
Bull JG, Saunders DE, Clark CA. Discrimination of paediatric brain tumours using apparent diffusion coefficient histograms. Eur Radiol. 2012;22:447–57.
Vajapeyam S, Brown D, Johnston PR, Ricci KI, Kieran MW, Lidov HGW, Poussaint TY. Multiparametric Analysis of Permeability and ADC Histogram Metrics for Classification of Pediatric Brain Tumors by Tumor Grade. AJNR Am J Neuroradiol. 2018;39:552–7.
Novak J, Zarinabad N, Rose H, Arvanitis T, MacPherson L, Pinkey B, Oates A, Hales P, Grundy R, Auer D, Gutierrez DR, Jaspan T, Avula S, Abernethy L, Kaur R, Hargrave D, Mitra D, Bailey S, Davies N, Clark C, Peet A. Classification of paediatric brain tumours by diffusion weighted imaging and machine learning. Sci Rep. 2021;11:2987.
Rodriguez Gutierrez D, Awwad A, Meijer L, Manita M, Jaspan T, Dineen RA, Grundy RG, Auer DP. Metrics and textural features of MRI diffusion to improve classification of pediatric posterior fossa tumors. AJNR Am J Neuroradiol. 2014;35:1009–15.
Koeller KK, Rushing EJ. From the archives of the AFIP: pilocytic astrocytoma: radiologic-pathologic correlation. Radiographics. 2004;24:1693–708.
Bornhorst M, Frappaz D, Packer RJ. Pilocytic astrocytomas. Handb Clin Neurol. 2016;134:329–44.
Duc NM, Huy HQ. Magnetic resonance imaging features of common posterior fossa brain tumors in children: a preliminary vietnamese study. Open Access Maced J Med Sci. 2019;7:2413–8.
Rasalkar DD, Chu WC, Paunipagar BK, Cheng FW, Li CK. Paediatric intra-axial posterior fossa tumours: pictorial review. Postgrad Med J. 2013;89:39–46.
Houghton E, Sethi B. Paediatric posterior fossa tumours: a pictorial review. Clin Radiol. 2020;75:e22.
Millard NE, De Braganca KC. Medulloblastoma. J Child Neurol. 2016;31:1341–53.
Perreault S, Ramaswamy V, Achrol AS, Chao K, Liu TT, Shih D, Remke M, Schubert S, Bouffet E, Fisher PG, Partap S, Vogel H, Taylor MD, Cho YJ, Yeom KW. MRI surrogates for molecular subgroups of medulloblastoma. AJNR Am J Neuroradiol. 2014;35:1263–9.
Meyers SP, Khademian ZP, Biegel JA, Chuang SH, Korones DN, Zimmerman RA. Primary intracranial atypical teratoid/rhabdoid tumors of infancy and childhood: MRI features and patient outcomes. AJNR Am J Neuroradiol. 2006;27:962–71.
Koral K, Gargan L, Bowers DC, Gimi B, Timmons CF, Weprin B, Rollins NK. Imaging characteristics of atypical teratoid-rhabdoid tumor in children compared with medulloblastoma. AJR Am J Roentgenol. 2008;190:809–14.
Han L, Qiu Y, Xie C, Zhang J, Lv X, Xiong W, Wang W, Zhang X, Wu P. Atypical teratoid/rhabdoid tumors in adult patients: CT and MR imaging features. AJNR Am J Neuroradiol. 2011;32:103–8.
Funding
No funds, grants, or other support were received.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Fabrício Guimarães Gonçalves, Alireza Zandifar, Jorge Du Ub Kim, Adarsh Ghosh, Luis Octavio Tierradentro-García and Dmitry Khrichenko. The first draft of the manuscript was written by Fabrício Guimarães Gonçalves, Alireza Zandifar, Jorge Du Ub Kim and Luis Octavio Tierradentro-Garcia. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
F.G. Gonçalves, A. Zandifar, J.D. Ub Kim, L.O. Tierradentro-García, A. Ghosh, D. Khrichenko, S. Andronikou and A. Vossough declare that they have no competing interests.
Ethical standards
This retrospective study was approved by our Institutional Review Board and is HIPPA compliant; a waiver for consent was granted. For this article no studies with human participants or animals were performed by any of the authors. All studies mentioned were in accordance with the ethical standards indicated in each case. This retrospective study was performed after consultation with the institutional ethics committee and in accordance with national legal requirements.
Additional information
Code Availability
Not applicable
Rights and permissions
About this article
Cite this article
Gonçalves, F.G., Zandifar, A., Ub Kim, J.D. et al. Application of Apparent Diffusion Coefficient Histogram Metrics for Differentiation of Pediatric Posterior Fossa Tumors. Clin Neuroradiol 32, 1097–1108 (2022). https://doi.org/10.1007/s00062-022-01179-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-022-01179-6