Abstract
Purpose
By increasing lung volume and decreasing respiration-induced tumour motion amplitude, administration of continuous positive airway pressure (CPAP) during stereotactic ablative radiotherapy (SABR) could allow for better sparing of the lungs and heart. In this study, we evaluated the effect of CPAP on lung volume, tumour motion amplitude and baseline shift, as well as the dosimetric impact of the strategy.
Methods
Twenty patients with lung tumours referred for SABR underwent 4D-computed tomography (CT) scans with and without CPAP (CPAP/noCPAP) at two timepoints (T0/T1). First, CPAP and noCPAP scans were compared for lung volume, tumour motion amplitude, and baseline shift. Next, CPAP and noCPAP treatment plans were computed and compared for lung dose parameters (mean lung dose (MLD), lung volume receiving 20 Gy (V20Gy), 13 Gy (V13Gy), and 5 Gy (V5Gy)) and mean heart dose (MHD).
Results
On average, CPAP increased lung volume by 8.0% (p < 0.001) and 6.3% (p < 0.001) at T0 and T1, respectively, but did not change tumour motion amplitude or baseline shift. As a result, CPAP administration led to an absolute decrease in MLD, lung V20Gy, V13Gy and V5Gy of 0.1 Gy (p = 0.1), 0.4% (p = 0.03), 0.5% (p = 0.04) and 0.5% (p = 0.2), respectively, while having no significant influence on MHD.
Conclusions
In patients referred for SABR for lung tumours, CPAP increased lung volume without modifying tumour motion or baseline shift. As a result, CPAP allowed for a slight decrease in radiation dose to the lungs, which is unlikely to be clinically significant.
Zusammenfassung
Zielsetzung
Aus der Erhöhung des Lungenvolumens und der Verringerung der atembedingten Tumorbewegungsamplitude beim Einsatz des kontinuierlichen positiven Atemwegsdrucks (CPAP „continuous positive airway pressure“) während der stereotaktischen ablativen Strahlentherapie (SABR „stereotactic ablative radiotherapy“) könnte ein erhöhter Schutz von Lunge und Herz resultieren. Diese Studie untersucht die Auswirkung der CPAP auf Lungenvolumen, Tumorbewegungsamplitude und „baseline shift“ sowie deren Einfluss auf die Dosisverteilung.
Methoden
Von 20 Patienten mit Lungentumoren, für die eine SABR indiziert ist, wurden zu zwei verschiedenen Zeitpunkten (T0/T1) 4‑D-Computertomographie-(CT-)Aufnahmen mit und ohne CPAP (CPAP/noCPAP) angefertigt. Zuerst wurden die CPAP- und noCPAP-Aufnahmen hinsichtlich Lungenvolumen, Tumorbewegungsamplitude und „baseline shift“ verglichen. Anschließend wurden CPAP- und noCPAP-Bestrahlungspläne erstellt und bezüglich der Dosisverteilung auf Herz und Lunge gegenübergestellt.
Ergebnisse
Im Durchschnitt vergrößerte CPAP das Lungenvolumen zu T0 und T1 um 8% (p < 0,001) bzw. 6,3% (p < 0,001), ohne Tumorbewegungsamplitude und „baseline shift“ zu verändern. Im Ergebnis verringerte die CPAP die mittlere Strahlendosis auf die Lunge um 0,1 Gy (p = 0,1) und die Volumenparameter V20Gy, V13Gy und V5Gy jeweils um 0,4% (p = 0,03), 0,5% (p = 0,04) und 0,5% (p = 0,2), ohne die Strahlendosis auf das Herz zu beeinflussen.
Schlussfolgerung
Bei Patienten, deren Lungentumor einer SABR unterzogen wurde, vergrößerte die CPAP das Lungenvolumen, ohne die Tumorbewegungsamplitude oder den „baseline shift“ zu beeinflussen. Infolgedessen wurde die Strahlendosis auf die Lunge leicht verringert, deren klinische Relevanz ist jedoch unwahrscheinlich.
Similar content being viewed by others
Introduction
Radiation therapy (RT) has long been recognised as one of the main treatment modalities in patients with lung cancer [1]. In recent years, major technological advancements such as intensity-modulated radiation therapy (IMRT) and image-guided radiation therapy (IGRT) have enabled the delivery of highly conformal treatments such as stereotactic ablative radiotherapy (SABR). This strategy consists of the delivery of a small number of high-dose fractions and is now considered as standard treatment in early-stage lung cancer for inoperable patients or patients refusing surgery. In these patients, SABR achieves impressive local control rates of more than 90% at 5 years [2] with low toxicity. Currently, SABR is only considered for the treatment of small tumours (<5 cm), for fear of unacceptable treatment toxicity in patients with larger tumours. In this context, novel strategies might allow to further decrease the treatment toxicity and apply SABR to a wider range of tumours.
A key characteristic of lung tumours is their mobile nature. On one hand, these tumours can move with breathing, and this motion can vary in amplitude, frequency and regularity over time [3]. On the other hand, they can display spatial shifts relative to the anatomy (or baseline shifts) over the course of the treatment [4]. In order to ensure correct dose coverage of the tumour, both respiration-induced tumour motion and baseline shifts should be taken into account. This can be simply done by including them in the planning target volume (PTV) margin, along with the other geometrical uncertainties (e.g. setup errors). However, this inevitably leads to larger irradiated volumes and futile dose to the healthy tissues surrounding the tumour. Several strategies have been developed to mitigate tumour motion and thereby reduce the irradiated volume. Among the most frequently mentioned are respiratory-gated RT and real-time tumour tracking. However, both these techniques necessitate complex and dedicated equipment. Another possible strategy is the use of repeated voluntary breath-holds, but these are usually of short duration (around 20 s) and largely depend on patient compliance.
Recently, the administration of continuous positive airway pressure (CPAP) during RT delivery was presented as a simple solution to reduce treatment toxicity [5]. As its name implies, CPAP consists of the administration of positive pressure to the airways during the entire respiratory cycle. This was shown to lead to two potential benefits in the context of lung cancer RT. First, by increasing the pressure in the airways, CPAP could increase lung volume and hence displace part of the healthy lung tissue and the heart out of the treatment field [5]. Second, in some patients, CPAP could decrease respiration-induced tumour motion amplitude and, therefore, the size of the PTV [5]. This reduction was attributed to the fact that as lung volume increases, the diaphragm flattens and shows reduced respiratory motion. As a result of both changes in lung volume and tumour motion amplitude, the administration of CPAP during RT promisingly resulted in reduced dose to the lungs and the heart based on pre-treatment imaging [5].
However, RT treatments are usually delivered in a fractionated manner, and it is not known whether the effect of CPAP on lung volume and tumour motion amplitude is reproducible over time. Notably, different levels of lung inflation from one day to another might result in baseline shifts of the tumour. As part of the RT treatment, the patient is positioned before each fraction based on an image of the day (e.g. megavoltage CT [MVCT] or cone beam CT [CBCT]). For lung tumours, this alignment is usually centred either on the tumour or on a stable landmark (e.g. carina or spine). In both situations, baseline shifts might jeopardise the quality of the treatment. In the first case, a shift of the tumour towards an adjacent critical structure (e.g. the spinal cord) might result in overdosing this structure. In the second case, a shift of the tumour away from the anatomical landmark might result in partly missing the tumour.
In the present study, we performed repeated 4D-CT with and without CPAP in patients with lung tumours, and evaluated the effect of CPAP on lung volume, tumour motion amplitude and baseline shift, as well as the dosimetric impact of the strategy.
Materials and methods
Patient selection
From July 2015 to March 2017, 20 patients with stage I lung tumours or lung metastases referred for stereotactic ablative radiotherapy (SABR) were prospectively included in this study (Table 1). Patients able to understand the instructions related to CPAP and with tumours expected to move significantly (apical tumours excluded) were eligible. One of the patients had two treatments 1 year apart and was recruited twice (#6 and #14). A total of 24 tumours were treated. Histological confirmation was available in 13 patients, who all had primary lung tumours. In other patients, malignancy of the pulmonary nodule was suspected based on imaging features, e.g. morphologic characteristics of malignancy, growth on consecutive CT scans and/or hypermetabolism on positron emission tomography (PET)/CT.
Image acquisition
Patients underwent 4D-CT scans with and without CPAP at two time-points: during the RT simulation session (T0) and on the first day of treatment (T1), resulting in four 4D-CT scans per patient: CPAPT0, noCPAPT0, CPAPT1 and noCPAPT1.
Regarding CPAP administration, patients underwent a short training session (approximately 15 min) with an experienced respiratory physiotherapist before the first imaging session. They were fitted with a nasal mask, connected to the CPAP device (SmartAir Plus, Airox, Pau, France) and airway pressure was set according to patient comfort.
All acquisitions were performed on a big bore CT scanner (Aquilion LB, Toshiba Medical Systems, Tokyo, Japan). Patients were immobilised in treatment position using a half-body vacuum bag (Orfit Industries NV, Wijnegem, Belgium), and were audio-coached to ensure regular breathing [3]. The respiratory signal was recorded using an optical tracking system (GateCT, VisionRT, London, UK), and the scanner helical pitch was set accordingly. 4D-CT scans covered the thoracic region (slice thickness: 2 mm) and were binned into ten equally distributed temporal frames.
Image reconstruction
Following each 4D-CT acquisition, an average CT was first computed by averaging the voxel density values over the ten CT frames. Next, a time-averaged mid-position (MidP) CT was generated from deformable registration of the ten CT frames using homemade software (REGGUI) [6], as previously described [7]. The MidP image represents the anatomy in its time-weighted mean position over the respiratory cycle and is visually sharp, in contrast to the average CT which is blurred by respiratory motion.
The amplitude of respiration-induced tumour motion was automatically computed during the MidP generation process. To this end, a point of interest was defined at the tumour level on the end-exhale CT frame. This point was then propagated to the nine other CT frames using deformable registration, and the maximal distance between the ten resulting points was computed.
Image analysis
For each patient, noCPAP and CPAP images were compared as follows:
First, the gross tumour volume (GTV) and the lungs were delineated by an experienced radiation oncologist using MIM Maestro version 6 (MIM Software, Cleveland, OH, USA).
-
GTV. The GTV was manually delineated using a standard lung window (level: −600, window: 1600) on the pre-treatment MidP CT (noCPAPT0), and propagated to the three other MidP CT (CPAPT0, noCPAPT1, and CPAPT1) using rigid registration centred on the tumour.
-
Lungs. The lungs were automatically segmented on each MidP CT, and a manual correction was performed when necessary (e.g. if a main bronchus or the diaphragm was initially included in the automatically segmented lung volume).
Next, the effect of CPAP on lung volume, tumour motion amplitude and baseline shift was evaluated.
-
Lung volume and tumour motion amplitude. Both parameters were compared between noCPAP and CPAP scans at both T0 and T1 time-points.
-
Baseline shift. Rigid registration based on the bony anatomy was performed between noCPAPT0 and noCPAPT1 MidP CT, and between CPAPT0 and CPAPT1 MidP CT. Next, the distance between GTV centroids was compared between both image pairs.
Treatment planning
For each patient, treatment planning was performed on noCPAPT0 and CPAPT0 images, and treatment plans were compared as follows:
First, the planning target volume (PTV) and organs at risk (OARs) were delineated on each MidP CT, and were rigidly propagated to the corresponding average CT.
-
PTV. The PTV was generated by volumetric expansion of the GTV. The PTV margin was computed using van Herk’s formula [8] as previously described [7], i.e.
$$\begin{aligned}\mathrm{M}_{\text{PTV}} =& 2.5\sqrt{\left(\Sigma _{\text{TM}}^{2}+\Sigma _{\text{BL}}^{2}+\Sigma _{\text{SETUP}}^{2}+\Sigma _{\mathrm{D}}^{2}\right)}\\ & +1.64\sqrt{\left(\sigma _{\text{TM}}^{2}+\sigma _{\text{BL}}^{2}+\sigma _{\text{SETUP}}^{2}+\sigma _{\mathrm{p}}^{2}\right)}-1.64\sigma _{p}\,,\end{aligned}$$where Σ and σ are the standard deviations of the systematic and random errors, respectively. The subscripts TM, BL, SETUP, D and p refer to tumour motion, baseline shift, patient setup, delineation and penumbra, respectively. All standard deviations were set in agreement with the literature [9,10,11], except σTM, which was defined according to the amplitude of tumour motion measured in the 4D-CT [11, 12], and σP which was specifically computed for our TomoTherapy (TomoTherapy Inc., Madison, WI, USA) system. The coefficients 2.5 and 1.64 in the formula ensure that the GTV receives at least 95% of the prescribed dose for 90% of the patients.
-
OARs. In addition to the lungs, the brachial plexus, bronchial tree, chest wall, oesophagus, great vessels, heart and spinal cord were delineated.
Next, treatment plans were computed on the average CT by an experienced medical physicist using the TomoTherapy Treatment Planning System version 5.1.0.4. The physicist was blinded regarding the status of the CT (noCPAP or CPAP). The dose regimen was chosen according to the location of the tumour (Table 1). Dose prescription was defined as the minimum dose received by 95% of the PTV. Treatment plans were generated using a fine dose grid, a field width ranging from 1.0 to 5.1 cm, a pitch ranging from 0.142 to 0.215 and a modulation factor ranging from 1.2 to 2.
Finally, dose parameters for the lungs (mean lung dose [MLD], lung volume receiving 20 Gy [V20Gy], 13 Gy [V13Gy], and 5 Gy[V5Gy]), and the heart (mean heart dose [MHD]) were compared between noCPAP and CPAP plans. Similarly, Paddick conformity indexes (CIPaddick) [13] were computed and compared, i.e., \(\text{CI}_{\text{Paddick}}=\text{TV}_{\text{PI}}^{2}/(\text{TV}\times \text{PI})\), where TVPI is the target volume (TV) within the prescribed isodose volume (PI). For a perfect plan, TVPI = PI = TV, resulting in CIPaddick = 1.
Statistical analysis
The effect of CPAP on lung volume, tumour motion amplitude and baseline shift was analysed using a generalised linear model with two intra-patient effects (noCPAP/CPAP and T0/T1). The effect of CPAP on dose parameters and conformity indexes was analysed using Student’s t-test. Data are reported as mean with 95% confidence interval (CI), and t-test results are reported as mean difference with standard error of the mean (SEM). SPSS Statistical Software Version 23 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses and graphic illustrations. All tests were two-tailed and a p-value lower than 0.05 was considered as significant.
Results
CPAP was well-tolerated in all but one (#13) of the patients. Airway pressure was set to an average value of 6.9 cm H2O (95% CI: [6.5–7.2]). The average time interval between T0 and T1 time-points was 10 days (95% CI: [8–11]). The average volume of the GTV was 4.4 mL (95% CI: [2.4–6.4]).
Image analysis
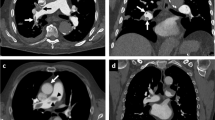
CPAP increased lung volume by 296 mL (SEM: 73; p < 0.001) and 243 mL (SEM: 52; p < 0.001), which corresponds to a mean relative increase of 8.0% (SEM: 2.2) and 6.3% (SEM: 1.3) at T0 and T1 time-points, respectively. The change in lung volume induced by CPAP showed a wide inter-patient variability (Fig. 1 and Table 2). For example, while some of the patients showed a large increase in lung volume at T0 (#16, #18 and #19; Fig. 2), two of the patients (#10 and #13) displayed a slight decrease, for which there is no obvious physiological explanation apart from anxiety (patient #13 was the one who did not tolerate CPAP well). Overall, the change in lung volume induced by CPAP at T0 was similar to T1 (interaction F = 0.31, p = 0.56). However, the intra-patient reproducibility was relatively low (Fig. 1).
In some patients (e.g. #16, #18 and #19), continuous positive airway pressure (CPAP) induced a large increase in lung volume. The noCPAPT0 midposition (MidP) CT, the CPAPT0 MidP CT and their fusion are displayed on the first, second and third rows, respectively. Lungs are delineated with a blue line
The effect of CPAP on tumour motion also showed a wide inter-patient variability. When considering all patients together, CPAP did not significantly change tumour motion amplitude at T0 (p = 0.45) or T1 (p = 0.14). Additionally, CPAP did not change baseline shift (p = 0.69; Table 2).
Treatment planning
CPAP administration led to a slightly smaller PTV in 13 out of the 20 patients, as a result of smaller tumour motion amplitude with CPAP in these patients (Table 3). Considering all patients together, this difference in PTV size due to CPAP was not significant (p = 0.3). Dose conformity assessed with Paddick conformity index was similar between CPAP (CIPaddick = 0.73) and noCPAP plans (CIPaddick = 0.73; p = 0.4).
Overall, CPAP administration only led to a slight decrease in lung dose parameters (Table 3). The mean lung dose (MLD) was reduced by 0.1 Gy (p = 0.1), which was mainly due to dose reduction to the lung where the tumour was located. Indeed, mean dose delivered to the homolateral lung was reduced by 0.3 Gy (SEM: 0.1; p < 0.01), while mean dose delivered to the contralateral lung was similar (p = 0.8). When analysing patients separately, CPAP led to an increase in MLD in five of the patients. This was due to a detrimental effect of CPAP on lung volume in two patients (#10 and 13), and on tumour motion amplitude in the three others (#14, #15 and #17). Lung V20Gy, V13Gy and V5Gy were reduced by 0.4% (SEM: 0.2; p = 0.03), 0.5% (SEM: 0.2; p = 0.04) and 0.5% (SEM: 0.4; p = 0.2), respectively.
Overall, CPAP had negligible influence on the mean heart dose (MHD; p = 0.7) (Table 3). Nevertheless, two patients had significantly higher MHD when administered CPAP, which was attributable either to the fact that the lung inflation induced by CPAP moved the tumour towards the heart (#10) or to the fact that CPAP increased the tumour motion amplitude and therefore the size of the PTV (#17).
Discussion
In this study, we performed repeated 4D-CT with and without CPAP in patients undergoing SABR for lung tumours. The administration of CPAP resulted in increased lung volume, without modifying tumour motion amplitude and baseline shift. Disappointingly, CPAP administration only led to a slight reduction in lung dose, which is unlikely to be clinically relevant.
Schematically, lung dose depends on two factors: lung volume and PTV size (which depends directly on tumour motion amplitude). If the former increases and the latter decreases, lung dose parameters should be improved, and vice versa. First, in our study, even patients who experienced large increases in lung volume with CPAP showed a modest decrease in lung dose. A possible explanation is that we enrolled patients referred for SABR, hence with small tumours and already receiving low dose to the lungs. Next, some of the patients in our study showed an increase in tumour motion amplitude with CPAP, for which we do not have any obvious explanation.
In the past, a single study has evaluated the use of CPAP during RT [5]. This study evaluated the repercussion of CPAP on lung volume and tumour motion amplitude in ten patients with lung tumours undergoing SABR. In these patients, the administration of CPAP resulted in a mean increase in lung volume of 915 mL (or 32%), and a mean decrease in tumour motion amplitude of approximately 5 mm. As a result, the mean lung and heart doses were reduced by 22% and 29%, respectively.
The smaller effects observed in our study might be due to its limitations. First, patients in the previous study received higher CPAP pressures (10 to 15 cm H2O) and were administrated CPAP for a longer time before imaging (at least 1 h) than our patients. Next, we used a nasal mask rather than a full-face mask. Therefore, some of our patients could have opened their mouth during the administration of CPAP, thereby lessening its effect. Moreover, we used the same audio-coaching track for both noCPAP and CPAP acquisitions, which could have stabilised the tumour motion amplitude.
Further studies are needed to allow for better understanding of the effect of CPAP in the context of RT and for selection of the patients who could benefit from the strategy. As demonstrated in our study, the effect of CPAP on lung volume and tumour motion showed a wide inter-patient variability and a low intra-patient reproducibility. The former could be explained by differences in lung and chest cage compliances and in tumour location between patients. The latter could be due to day-to-day variations in the degree of bronchopulmonary obstruction. In our patient cohort, we did not identify predictive factors for the effect of CPAP. In future studies, we recommend using the methodology proposed by Goldstein et al. [5] (notably the higher airway pressure), which was more efficient for increasing lung volume and decreasing tumour motion amplitude, but whose reproducibility is still unknown. Future studies might be based on 4D-MRI, which has the advantage of being a non-ionising image modality. This could allow to test, for each patient, different levels or airway pressure, as well as the reproducibility of CPAP effects over several sessions. Importantly, the most important benefit which should be expected from CPAP administration during RT is the reduction in tumour motion amplitude (and thereby in the size of the PTV), rather than the increase in lung volume. Indeed, in the latter case, the consequential reduction in lung dose parameters might be partly artificial. Indeed, pulling away part of the lung out of the treatment field should theoretically provide a clinical benefit. However, inflating pulmonary alveoli which are already out of the treatment field will increase lung volume and improve dose parameters, but the resulting clinical effect is less straightforward.
Two other ventilation strategies might be more promising and reliable than CPAP when it comes to reducing respiratory-induced tumour motion: mechanical ventilation (MV) and high-frequency ventilation (HFV). Indeed, MV can be used to take control over breathing in conscious and unsedated patients. By imposing constant respiratory rate and volume parameters, MV allows for regularising the respiratory pattern [14], which might improve its reproducibility. Pushing the strategy one step further, MV and HFV could allow for decreasing or suppressing respiratory-induced tumour motion. On the one hand, MV might be used to impose a rapid shallow ventilation on the patient (high respiratory rate and small tidal volume), which should result in smaller tumour motion [14]; on the other hand, using a slow deep ventilation (low respiratory rate and large tidal volume) could yield periods of tumour immobility between the breathing cycles, which could be beneficial in the context of gated RT [14]. Similarly, HFV aims at freezing the tumour position during irradiation [15,16,17,18]. This is achieved through the administration of small boluses of air at high pressure and frequency (around 300 breaths per minute). By doing so, HFV provided good tumour immobilisation during the delivery of single-dose stereotactic irradiation to the liver [15] and the lung [16]. In these studies, HPJV was performed on anesthetised patients, but the strategy could also be applicable to conscious patients [17, 19]. These strategies are currently under investigation in our institution.
Conclusion
In summary, in patients referred for SABR for lung tumours, CPAP increased lung volume without modifying tumour motion amplitude or baseline shift. As a result, CPAP allowed for a slight decrease in radiation dose to the lungs which is unlikely to be clinically significant.
References
Vansteenkiste J, De Ruysscher D, Eberhardt WE et al (2013) Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 24(Suppl 6):vi89–vi98
Verstegen NE, Lagerwaard FJ, Hashemi SM et al (2015) Patterns of disease recurrence after SABR for early stage non-small-cell lung cancer: optimizing follow-up schedules for salvage therapy. J Thorac Oncol 10:1195–1200
Goossens S, Senny F, Lee JA et al (2014) Assessment of tumor motion reproducibility with audio-visual coaching through successive 4D CT sessions. J Appl Clin Med Phys 15:4332
Kwint M, Conijn S, Schaake E et al (2014) Intra thoracic anatomical changes in lung cancer patients during the course of radiotherapy. Radiother Oncol 113:392–397
Goldstein JD, Lawrence YR, Appel S et al (2015) Continuous positive airway pressure for motion management in stereotactic body radiation therapy to the lung: a controlled pilot study. Int J Radiat Oncol Biol Phys 93:391–399
Janssens G, Jacques L, Orban de Xivry J et al (2011) Diffeomorphic registration of images with variable contrast enhancement. Int J Biomed Imaging 2011:891585
Wanet M, Sterpin E, Janssens G et al (2014) Validation of the mid-position strategy for lung tumors in helical TomoTherapy. Radiother Oncol 110:529–537
van Herk M, Remeijer P, Rasch C et al (2000) The probability of correct target dosage: dose-population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol Biol Phys 47:1121–1135
Bissonnette JP, Franks KN, Purdie TG et al (2009) Quantifying interfraction and intrafraction tumor motion in lung stereotactic body radiotherapy using respiration-correlated cone beam computed tomography. Int J Radiat Oncol Biol Phys 75:688–695
Sonke JJ, Lebesque J, van Herk M (2008) Variability of four-dimensional computed tomography patient models. Int J Radiat Oncol Biol Phys 70:590–598
Wolthaus JW, Schneider C, Sonke JJ et al (2006) Mid-ventilation CT scan construction from four-dimensional respiration-correlated CT scans for radiotherapy planning of lung cancer patients. Int J Radiat Oncol Biol Phys 65:1560–1571
Wolthaus JW, Sonke JJ, van Herk M et al (2008) Comparison of different strategies to use four-dimensional computed tomography in treatment planning for lung cancer patients. Int J Radiat Oncol Biol Phys 70:1229–1238
Paddick I (2000) A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg 93(Suppl 3):219–222
Parkes MJ, Green S, Stevens AM et al (2016) Reducing the within-patient variability of breathing for radiotherapy delivery in conscious, unsedated cancer patients using a mechanical ventilator. Br J Radiol 89:20150741
Fritz P, Kraus HJ, Dolken W et al (2006) Technical note: gold marker implants and high-frequency jet ventilation for stereotactic, single-dose irradiation of liver tumors. Technol Cancer Res Treat 5:9–14
Fritz P, Kraus HJ, Muhlnickel W et al (2010) High-frequency jet ventilation for complete target immobilization and reduction of planning target volume in stereotactic high single-dose irradiation of stage I non-small cell lung cancer and lung metastases. Int J Radiat Oncol Biol Phys 78:136–142
Peguret N, Ozsahin M, Zeverino M et al (2016) Apnea-like suppression of respiratory motion: first evaluation in radiotherapy. Radiother Oncol 118:220–226
Santiago A, Jelen U, Ammazzalorso F et al (2013) Reproducibility of target coverage in stereotactic spot scanning proton lung irradiation under high frequency jet ventilation. Radiother Oncol 109:45–50
Prior JO, Peguret N, Pomoni A et al (2016) Reduction of respiratory motion during PET/CT by pulsatile-flow ventilation: a first clinical evaluation. J Nucl Med 57:416–419
Acknowledgements
We would like to thank Valérie Lacroix who provided the original idea for this article and Karolin Schneider who translated the abstract into German.
Funding
This study was funded by the Fonds de la Recherche Scientifique—FNRS—Télévie (grant n°7.4572.13F) and the Fondation Contre le Cancer (grant n°2014-086).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
D. Di Perri, A. Colot, A. Delor, R. Ghoul, G. Janssens, V Lacroix, P. Matte, A. Robert, K. Souris and X. Geets declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (registration number of the ethical approval: B403201524475) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Di Perri, D., Colot, A., Delor, A. et al. Effect of continuous positive airway pressure administration during lung stereotactic ablative radiotherapy: a comparative planning study. Strahlenther Onkol 194, 591–599 (2018). https://doi.org/10.1007/s00066-018-1278-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-018-1278-2