Abstract
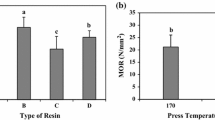
Considering the importance of urea–formaldehyde (UF) resins in the wood industry, this work reports on a new bio-based modification of UF resins. The use of 5-hydroxymethyl furfural (HMF) is motivated by the current concerns about the effects of formaldehyde on human health. UF and urea–HMF–formaldehyde (UHF) resins were synthesized by an alkaline-acid method and characterized by FTIR, thermogravimetric analysis, and differential scanning calorimetry. The UHF, as a newly modified polymeric resin, was thermally characterized, and it was found that its thermo-stability and char yield was improved. In order to investigate the performance of the UHF, the preparation of particleboards with the UHF as adhesive, as well as its film formation ability have been studied. The UHF films formed on wood panels were uniform without any crack. Film formation ability of the UHF resin was improved due to the presence of more hydroxyl groups as well as furan rings of the HMF moieties resulting in more activated groups to be bonded by wood. Furthermore, formaldehyde release of the particleboards bonded by UHF was significantly lower than that of which bonded by the UF resin. Lab particleboards using the UHF resins showed higher modulus of rupture, modulus of elasticity, and internal bond compared to boards with UF resins, as well as lower water absorption and thickness swelling. Based on these results UHF resin can be considered as a possible candidate as adhesive for wood and wood based panels.







Similar content being viewed by others
References
Abdullah ZA, Park BD (2010) Influence of acrylamide copolymerization of urea formaldehyde resin adhesives to their chemical structure and performance. J Appl Polym Sci 117:3181–3186
Ahamad T, Alshehri SM (2014) Thermal degradation and evolved gas analysis: a polymeric blend of urea formaldehyde (UF) and epoxy (DGEBA) resin. Arab J Chem 7:1140–1147
Basta AH, El-Saied H, Winandy JE, Sabo R (2011) Preformed amide-containing biopolymer for improving the environmental performance of synthesized urea–formaldehyde in agro-fiber composites. J Polym Environ 19:405–412
Birkeland MJ, Lorenz L, James M, Frihart CR (2010) Determination of native (wood derived) formaldehyde by the desiccator method in particleboards generated during panel production. Holzforschung 64:429–433
Bobrowski A, Grabowska B (2012) The impact of temperature on furan resin. Metall Foundry Eng 38:73–80
Bono A, Beng YK, Siambun NJ (2003) Melamine–urea–formaldehyde (MUF) resin: the effect of the number of reaction stages and mole ratio on resin properties. J Teknol 38:43–52
Christjanson P, Siimer K, Pehk T, Lasn I (2002) Structural changes in urea–formaldehyde resins during storage. Eur J Wood Prod 60:379–384
Christjanson P, Pehk T, Siimer K (2006) Hydroxymethylation and polycondensation reactions in urea–formaldehyde resin synthesis. J Appl Polym Sci 100:1673–1680
Dim PE (2011) Application of keratin-modified urea–formaldehyde resin for bonding particleboard. Aust J Basic Appl Sci 5:196–200
Ebewele RO (1995) Differential scanning calorimetry and dynamic mechanical analysis of amine-modified urea–formaldehyde adhesives. J Appl Polym Sci 58:1689–1700
Esmaeili N, Zohuriaan-Mehr MJ, Bouhendi H, Bagheri-Marandi G (2016) HMF synthesis in aqueous and organic media under ultrasonication, microwave irradiation and conventional heating. Korean J Chem Eng 33:1964–1970
Ferra J (2010) Optimization of urea–formaldehyde resins for the manufacture of wood-based panels. PhD thesis dissertation, University of Porto, pp 3–27
Ferra MM, Henriques A, Mendes M, Costa RN, Carvalho LH, Magalhaes F (2012) Comparison of UF synthesis by alkaline-acid and strongly acid processes. J Appl Polym Sci 123:1764–1772
Fink JK (2005) Reactive polymers fundamentals and applications, chaps 5 and 7. William Andrew Pub, Norwich
Gancarz R (1995) Nucleophilic addition to carbonyl compounds. Tetrahedron 51:10627–10632
Gandini A (2010) Biocatalysis in polymer chemistry. Wiley, Singapore, pp 1–34
Gao Z, Wang X, Wan H, Liu Y (2008) Curing characteristics of urea–formaldehyde resin in the presence of various amounts of wood extracts and catalysts. J Appl Polym Sci 107:1555–1562
Han TL, Kumar RN, Rozman HD, Wan Daud WR (2008) Influence of process variables on the reactivity of low formaldehyde emission urea–formaldehyde resin. Polym Plast Technol Eng 47:551–557
Jiang X, Li C, Chi Y, Yan J (2010) TG-FTIR study on urea–formaldehyde resin residue during pyrolysis and combustion. J Hazard Mater 173:205–210
Kandelbauer A, Despres A, Pizzi A, Taudes I (2007) Testing by Fourier transform infrared species variation during melamine–urea–formaldehyde resin preparation. J Appl Polym Sci 106:2192–2197
Li H, Zhang Y, Zeng X (2009) Two-step synthesis and characterization of urea–isobutyraldehyde–formaldehyde resins. Prog Org Coat 66:167–172
Liu Y, Tian Y, Zhao G, Sun Y, Zhu F, Gao Y (2008) Synthesis of urea–formaldehyde resin by melt condensation polymerization. J Polym Res 15:501–505
No BY, Kim MG (2007) Evaluation of melamine-modified urea–formaldehyde resins as particleboard binders. J Appl Polym Sci 106:30–37
Paiva NT, Pereira J, Ferra JM, Cruz P, Carvalho L, Magalhaes FD (2012) Study of influence of synthesis conditions on properties of melamine–urea formaldehyde resins. Int Wood Prod J 3:51–57
Park BD, Kim JW (2008) Dynamic mechanical analysis of urea–formaldehyde resin adhesives with different formaldehyde-to-urea molar ratios. J Appl Polym Sci 108:2045–2051
Park BD, Chang Kang E, Yong Park J (2006) Effects of formaldehyde to urea mole ratio on thermal curing behavior of urea–formaldehyde resin and properties of particleboard. J Appl Polym Sci 101:1787–1792
Park B, Kang E, Park J (2008) Thermal curing behavior of modified urea–formaldehyde resin adhesives with two formaldehyde scavengers and their influence on adhesion performance. J Appl Polym Sci 110:1573–1580
Patel BK, Patel HS, Morekar MM (2013) Synthesis, characterization and glass reinforcement of dimethylolurea-3-aminophenol-epoxy resin curing systems. Polym Plast Technol Eng 52:332–336
Roumeli E, Papadopoulou E, Pavlidou E (2012) Synthesis, characterization and thermal analysis of urea–formaldehyde/nanoSiO2 resins. Thermochim Acta 527:33–39
Singh AP, Nuryawan A, Park BA (2013) Novel sample preparation method that enables ultrathin sectioning of urea–formaldehyde resin for imaging by transmission electron microscopy. Microsc Res 1:1–6
Sperling LH (2005) Introduction of polymer chemistry science. Wiley Press, New Jersey, pp 407–411
Taylor P, Hazarika A, Maji TK (2013) Study on the properties of wood polymer nanocomposites based on melamine formaldehyde–furfuryl alcohol copolymer and modified clay. J Wood Chem Technol 33:103–124
Tohmura S, Hse Y, Higuchi M (2000) Formaldehyde emission and high-temperature stability of cured urea–formaldehyde resins. J Wood Sci 46:303–309
Tumolva T, Kubouchi M, Aoki S, Sakai T (2009) Evaluating the carbon potential of furan resin-based green composites. In: 18th International conference on composite materials, Edinburgh, July 27–31
Van Putten R, Van Der Waal JC, De Jong E, Rasrendra CB, Heeres HJ, Vries JG (2013) Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem Rev 113:1499–1597
Zhang J, Chen H, Pizzi A, Li Y, Gao Q, Li J (2014) Characterization and application of urea–formaldehyde–furfural co-condensed resins as wood adhesives. Bioresources 9:6267–6276
Zorba T, Papadopoulou E, Hatjiissaak A, Paraskevopoulos KM, Chrissafis K (2008) Urea–formaldehyde resins characterized by thermal analysis and FTIR method. J Therm Anal Calorim 92:29–33
Acknowledgments
The authors are very much obliged to one of the reviewers due to his/her highly informative and deep comments causing evolutionary improvement of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Esmaeili, N., Zohuriaan-Mehr, M.J., Mohajeri, S. et al. Hydroxymethyl furfural-modified urea–formaldehyde resin: synthesis and properties. Eur. J. Wood Prod. 75, 71–80 (2017). https://doi.org/10.1007/s00107-016-1072-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00107-016-1072-8