Abstract
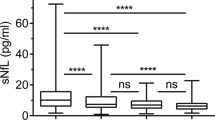
Fingolimod (FTY) is known to have multiple effects on the immune system and the central nervous system (CNS) in patients with multiple sclerosis (MS). In this study, we evaluated the immunological and neurobiological effects of FTY in MS. Blood and cerebrospinal fluid (CSF) samples were collected from 15 MS patients before first FTY administration and after 4 months of FTY therapy. Immunophenotyping and evaluation of sphingosine-1-phosphate (S1P), neurofilament light chain (NFL), S-100 and neuron-specific enolase (NSE) levels were conducted. After 4 months of FTY therapy, absolute cell count in CSF was decreased from 6.33 to 2.43 MPt/l, accompanied by decreases of CD3+ (2.22 to 0.65 MPt/l) and of CD4+ counts (1.60 to 0.39 MPt/l). In blood, CD3+ (1.05 to 0.09 GPt/l), CD4+ (0.80 to 0.02 GPt/l), CD8+ (0.23 to 0.04 GPt/l) and CD19+ (0.21 to 0.01GPt/l) cell counts were as well reduced. CD14+ cell count remained stable over the same period (0.24 to 0.26GPt/l). NFL and S1P levels in CSF and blood were reduced over time (NFL: CSF 1759 to 1359 pg/l, blood 8.42 to 7.36 pg/l; S1P: CSF 2.12 to 0.71 nmol/l, blood 392.1 to 312.9 nmol/l). Strong correlations between CSF and blood NFL levels were observed. Neuronal damage markers such as S-100 (1.86 to 1.69 μg/l) and NSE (9.53 to 8.67 μg/l) were reduced to a lesser degree than other markers. FTY exerted significant effects on immunological and neurobiological markers in the central and peripheral compartment. Decreases in levels of neuroinflammatory and neurodegenerative markers were already evident after 4 months of treatment. Four-month serum NFL level appears to be a useful marker for FTY efficacy that correlates well with changes in the CNS compartment.
Key messages
-
FTY has important immunological effects in both central and peripheral compartments.
-
Cellular effects of FTY effects are more pronounced in the blood than in the CSF.
-
FTY reduces S1P and NFL levels in CSF and serum.
-
Serum NFL appears to be a useful marker for FTY therapy.


Similar content being viewed by others
References
Thomas K, Proschmann U, Ziemssen T (2017) FTY hydrochloride for the treatment of relapsing remitting multiple sclerosis. Expert Opin Pharmacother 18:1649–1660
Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, Pelletier J, Capra R, Gallo P, Izquierdo G, Tiel-Wilck K, de Vera A, Jin J, Stites T, Wu S, Aradhye S, Kappos L (2010) Oral FTY or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362:402–415
Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, Burtin P (2010) A placebo-controlled trial of oral FTY in relapsing multiple sclerosis. N Engl J Med 362:387–401
Thomas K, Sehr T, Proschmann U, Rodriguez-Leal FA, Haase R, Ziemssen T (2017) FTY additionally acts as immunomodulator focused on the innate immune system beyond its prominent effects on lymphocyte recirculation. J Neuroinflammation 14(1):41
Chun J, Hartung HP (2010) Mechanism of action of oral FTY (FTY720) in multiple sclerosis. Clin Neuropharmacol 33:91–101
Mehling M, Brinkmann V, Antel J, Bar-Or A, Goebels N, Vedrine C, Kristofic C, Kuhle J, Lindberg RL, Kappos L (2008 Oct 14) FTY720 therapy exerts differential effects on T cell subsets in multiple sclerosis. Neurology 71(16):1261–1267
Claes N, Tessa Dhaeze., Judith Fraussen, Bieke Broux, Bart Van Wijmeersch, Piet Stinissen,Raymond Hupperts, Niels Hellings, Veerle Somers. Compositional changes of B and T cell subtypes during fingolimod treatment in multiple sclerosis patients: a 12-month follow-up study. PLoS One 2014; 9(10): e111115. Published online 2014 Oct 31. doi:
Kowarik MC, Pellkofer HL, Cepok S, Korn T, Kümpfel T, Buck D, Hohlfeld R, Berthele A, Hemmer B (2011) Differential effects of FTY (FTY720) on immune cells in the CSF and blood of patients with MS. Neurology. 76(14):1214–1221
Nakamura M, Matsuoka T, Chihara N, Miyake S, Sato W, Araki M, Okamoto T, Lin Y, Ogawa M, Murata M, Aranami T, Yamamura T (2014) Differential effects of fingolimod on B-cell populations in multiple sclerosis. Mult Scler 20(10):1371–1380
De Stefano N, Silva DG, Barnett MH (2017) Effect of FTY on brain volume loss in patients with multiple sclerosis. CNS Drugs 31(4):289–305
Kuhle J, Disanto G, Lorscheider J, Stites T, Chen Y, Dahlke F, Francis G, Shrinivasan A, Radue EW, Giovannoni G, Kappos LFTY (2015) CSF neurofilament light chain levels in relapsing-remitting multiple sclerosis. Neurology 84(16):1639–1643
Disanto G, Barro C, Benkert P, Naegelin Y, Schädelin S, Giardiello A, Zecca C, Blennow K, Zetterberg H, Leppert D, Kappos L, Gobbi C, Kuhle J (2017) Swiss multiple sclerosis cohort study group. Serum neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann Neurol 81(6):857–870
Piehl F, Kockum I, Khademi M, Blennow K, Lycke J, Zetterberg H, Olsson T (2017) Plasma neurofilament light chain levels in patients with MS switching from injectable therapies to FTY. Mult Scler 1:1352458517715132
Novakova L, Axelsson M, Khademi M, Zetterberg H, Blennow K, Malmeström C, Piehl F, Olsson T, Lycke J (2017) Cerebrospinal fluid biomarkers of inflammation and degeneration as measures of FTY efficacy in multiple sclerosis. Mult Scler 23(1):62–71
Kułakowska A, Zendzian-Piotrowska M, Baranowski M, Konończuk T, Drozdowski W, Górski J, Bucki R (2010) Intrathecal increase of sphingosine 1-phosphate at early stage multiple sclerosis. Neurosci Lett 477(3):149–152
Kułakowska A, Byfield FJ, Żendzian-Piotrowska M, Zajkowska JM, Drozdowski W, Mroczko B, Janmey PA, Buck R (2014) Increased levels of sphingosine-1-phosphate in cerebrospinal fluid of patients diagnosed with tick-borne encephalitis. J Neuroinflammation 11:193
Colombo E, Di Dario M, Capitolo E, Chaabane L, Newcombe J, Martino G, Farina C (2014) Fingolimod may support neuroprotection via blockade of astrocyte nitric oxide. Ann Neurol 76(3):325–337
Lee DH, Seubert S, Huhn K, Brecht L, Rötger C, Waschbisch A, Schlachetzki J, Klausmeyer A, Melms A, Wiese S, Winkler J, Linker RA (2017) Fingolimod effects in neuroinflammation: regulation of astroglial glutamate transporters? PLoS One 12(3):e0171552
Ziemssen T, Hillert J, Butzkueven H (2016) The importance of collecting structured clinical information on multiple sclerosis. BMC Med 14(1):81–81
Kuhle J, Barro C, Andreasson U, Derfuss T, Lindberg R, Sandelius Å, Liman V, Norgren N, Blennow K, Zetterberg H (2016) Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med 54(10):1655–1661
Wilson DH, Rissin DM, Kan CW, Fournier DR, Piech T, Campbell TG, Meyer RE, Fishburn MW, Cabrera C, Patel PP, Frew E, Chen Y, Chang L, Ferrell EP, von Einem V, McGuigan W, Reinhardt M, Sayer H, Vielsack C, Duffy DC (2016) The Simoa HD-1 analyzer: a novel fully automated digital immunoassay analyzer with single-molecule sensitivity and multiplexing. J Lab Autom 21(4):533–547
Min K, Yoo HS, Lee EY, Lee WJ, Ym L (2002) Simultaneous quantitative analysis of sphingoid base 1-phosphates in biological samples by o-phthalaldehyde precolumn derivatization after dephosphorylation with alkaline phosphatase. Anal Biochem 303:167–175
Lisa Lohmann, Claudia Janoschka, Andreas Schulte-Mecklenbeck, Svenja Klinsing, Lucienne Kirstein, Uta Hanning, Timo Wirth, Tilman Schneider-Hohendorf, Nicholas Schwab, Catharina C. Gross, Maria Eveslage, Sven G. Meuth, Heinz Wiendl and Luisa Klotz Immune cell profiling during switching from natalizumab to fingolimod reveals differential effects on systemic immune-regulatory networks and on trafficking of non-T cell populations into the cerebrospinal fluid—results from the ToFingo Successor Study. Front Immunol, 2018. https://doi.org/10.3389/fimmu.2018.01560
Grutzke B, Hucke S, Gross CC, Herold MV, Posevitz-Fejfar A, Wildemann BT et al (2015) Fingolimod treatment promotes regulatory phenotype and function of B cells. Ann Clin Transl Neurol 2(2):119–130
Nishihara H, Shimizu F, Sano Y, Takeshita Y, Maeda T, Abe M, Koga M, Kanda T (2015) Fingolimod prevents blood-brain barrier disruption induced by the sera from patients with multiple sclerosis. PLoS One 10(3):e0121488
Kuhle J, Disanto G, Lorscheider J, Stites T, Chen Y, Dahlke F, Francis G, Shrinivasan A, Radue EW, Giovannoni G, Kappos L (2015) Fingolimod and CSF neurofilament light chain levels in relapsing-remitting multiple sclerosis. Neurology 84(16):1639–1643
Novakova L, Axelsson M, Khademi M, Zetterberg H, Blennow K, Malmeström C, Piehl F, Olsson T, Lycke J (2017) Cerebrospinal fluid biomarkers of inflammation and degeneration as measures of fingolimod efficacy in multiple sclerosis. Mult Scler 23(1):62–71
Piehl F, Kockum I, Khademi M, Blennow K, Lycke J, Zetterberg H, Olsson T (2018) Plasma neurofilament light chain levels in patients with MS switching from injectable therapies to fingolimod. Mult Scler 24(8):1046–1054
Proia RL, Hla T (2015) Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy. J Clin Invest 125(4):1379–1387
Hunter SF, Bowen JD, Reder AT (2016) The direct effects of fingolimod in the central nervous system: implications for relapsing multiple sclerosis. CNS Drugs 30(2):135–147
Aoki M, Aoki H, Ramanathan R, Hait NC, Takabe K (2016) Sphingosine-1-phosphate signaling in immune cells and inflammation: roles and therapeutic potential. Mediat Inflamm 2016:8606878
Annunziata P, Cioni C, Masi G, Tassi M, Marotta G, Severi S (2018) Fingolimod reduces circulating tight-junction protein levels and in vitro peripheral blood mononuclear cells migration in multiple sclerosis patients. Sci Rep 8(1):15371
Katja Akgün, Nicole Kretschmann, Rocco Haase, Undine Proschmann, Hagen H. Kitzler, Heinz Reichmann, Tjalf Ziemssen, (2019) Profiling individual clinical responses by high-frequency serum neurofilament assessment in MS. Neurology - Neuroimmunology Neuroinflammation 6 (3):e555
Maxi Kaufmann, Rocco Haase, Undine Proschmann, Tjalf Ziemssen, Katja Akgün, (2018) Real World Lab Data: Patterns of Lymphocyte Counts in Fingolimod Treated Patients. Frontiers in Immunology 9
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
TS, RB and MZP have nothing to declare. KA received personal compensation from Novartis, Biogen Idec, Teva, Sanofi and Roche for the consulting service. TZ received personal compensation from Biogen Idec, Bayer, Novartis, Sanofi, Teva, and Synthon for the consulting services. Ziemssen received additional financial support for the research activities from Bayer, Biogen Idec, Novartis, Teva, and Sanofi Aventis.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sehr, T., Akgün, K., Proschmann, U. et al. Early central vs. peripheral immunological and neurobiological effects of fingolimod—a longitudinal study. J Mol Med 97, 1263–1271 (2019). https://doi.org/10.1007/s00109-019-01812-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-019-01812-x