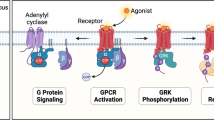
Abstract
Phylogenetic analysis of human G protein-coupled receptors (GPCRs) divides these transmembrane signaling proteins into five groups: glutamate, rhodopsin, adhesion, frizzled, and secretin families, commonly abbreviated as the GRAFS classification system. The adhesion GPCR (aGPCR) sub-family comprises 33 different receptors in humans. Majority of the aGPCRs are orphan receptors with unknown ligands, structures, and tissue expression profiles. They have a long N-terminal extracellular domain (ECD) with several adhesion sites similar to integrin receptors. Many aGPCRs undergo autoproteolysis at the GPCR proteolysis site (GPS), enclosed within the larger GPCR autoproteolysis inducing (GAIN) domain. Recent breakthroughs in aGPCR research have created new paradigms for understanding their roles in organogenesis. They play crucial roles in multiple aspects of organ development through cell signaling, intercellular adhesion, and cell–matrix associations. They are involved in essential physiological processes like regulation of cell polarity, mitotic spindle orientation, cell adhesion, and migration. Multiple aGPCRs have been associated with the development of the brain, musculoskeletal system, kidneys, cardiovascular system, hormone secretion, and regulation of immune functions. Since aGPCRs have crucial roles in tissue patterning and organogenesis, mutations in these receptors are often associated with diseases with loss of tissue integrity. Thus, aGPCRs include a group of enigmatic receptors with untapped potential for elucidating novel signaling pathways leading to drug discovery. We summarized the current knowledge on how aGPCRs play critical roles in organ development and discussed how aGPCR mutations/genetic variants cause diseases.



Similar content being viewed by others
Data availability
Since no datasets were used in this manuscript, data sharing policies are not applicable.
References
Bjarnadottir TK, Gloriam DE, Hellstrand SH, Kristiansson H et al (2006) Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics 88:263–273
Hauser AS, Chavali S, Masuho I, Jahn LJ et al (2018) Pharmacogenomics of GPCR drug targets. Cell 172(41–54):e19
Schioth HB, Fredriksson R (2005) The GRAFS classification system of G-protein coupled receptors in comparative perspective. Gen Comp Endocrinol 142:94–101
Baud V, Chissoe SL, Viegas-Pequignot E, Diriong S et al (1995) EMR1, an unusual member in the family of hormone receptors with seven transmembrane segments. Genomics 26:334–344
de la Lastra JM, Shahein YE, Garrido JJ, Llanes D (2003) Molecular cloning and structural analysis of the porcine homologue to CD97 antigen. Vet Immunol Immunopathol 93:107–115
Krasnoperov VG, Bittner MA, Beavis R, Kuang Y et al (1997) alpha-Latrotoxin stimulates exocytosis by the interaction with a neuronal G-protein-coupled receptor. Neuron 18:925–937
Lelianova VG, Davletov BA, Sterling A, Rahman MA et al (1997) Alpha-latrotoxin receptor, latrophilin, is a novel member of the secretin family of G protein-coupled receptors. J Biol Chem 272:21504–21508
Usui T, Shima Y, Shimada Y, Hirano S et al (1999) Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell 98:585–595
Nishimori H, Shiratsuchi T, Urano T, Kimura Y et al (1997) A novel brain-specific p53-target gene, BAI1, containing thrombospondin type 1 repeats inhibits experimental angiogenesis. Oncogene 15:2145–2150
Scholz N, Langenhan T, Schoneberg T (2019) Revisiting the classification of adhesion GPCRs. Ann N Y Acad Sci 1456:80–95
Krasnoperov V, Lu Y, Buryanovsky L, Neubert TA et al (2002) Post-translational proteolytic processing of the calcium-independent receptor of alpha-latrotoxin (CIRL), a natural chimera of the cell adhesion protein and the G protein-coupled receptor. Role of the G protein-coupled receptor proteolysis site (GPS) motif. J Biol Chem 277:46518–46526
Lin HH, Chang GW, Davies JQ, Stacey M et al (2004) Autocatalytic cleavage of the EMR2 receptor occurs at a conserved G protein-coupled receptor proteolytic site motif. J Biol Chem 279:31823–31832
Arac D, Boucard AA, Bolliger MF, Nguyen J et al (2012) A novel evolutionarily conserved domain of cell-adhesion GPCRs mediates autoproteolysis. EMBO J 31:1364–1378
Guan C, Cui T, Rao V, Liao W et al (1996) Activation of glycosylasparaginase. Formation of active N-terminal threonine by intramolecular autoproteolysis. J Biol Chem 271:1732–1737
Xu Q, Buckley D, Guan C, Guo HC (1999) Structural insights into the mechanism of intramolecular proteolysis. Cell 98:651–661
Chang GW, Stacey M, Kwakkenbos MJ, Hamann J et al (2003) Proteolytic cleavage of the EMR2 receptor requires both the extracellular stalk and the GPS motif. FEBS Lett 547:145–150
Volynski KE, Silva JP, Lelianova VG, Rahman A et al (2004) Latrophilin fragments behave as independent proteins that associate and signal on binding of LTX(N4C). EMBO J 23:4423–4433
Silva JP, Lelianova V, Hopkins C, Volynski KE et al (2009) Functional cross-interaction of the fragments produced by the cleavage of distinct adhesion G-protein-coupled receptors. J Biol Chem 284:6495–6506
Kishore A, Hall RA (2016) Versatile signaling activity of adhesion GPCRs. Handb Exp Pharmacol 234:127–146
Stoveken HM, Hajduczok AG, Xu L, Tall GG (2015) Adhesion G protein-coupled receptors are activated by exposure of a cryptic tethered agonist. Proc Natl Acad Sci U S A 112:6194–6199
Liebscher I, Schon J, Petersen SC, Fischer L et al (2014) A tethered agonist within the ectodomain activates the adhesion G protein-coupled receptors GPR126 and GPR133. Cell Rep 9:2018–2026
Henderson DJ, Long DA, Dean CH (2018) Planar cell polarity in organ formation. Curr Opin Cell Biol 55:96–103
Strutt D, Schnabel R, Fiedler F, Promel S (2016) Adhesion GPCRs govern polarity of epithelia and cell migration. Handb Exp Pharmacol 234:249–274
Curtin JA, Quint E, Tsipouri V, Arkell RM et al (2003) Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol 13:1129–1133
Langenhan T, Promel S, Mestek L, Esmaeili B et al (2009) Latrophilin signaling links anterior-posterior tissue polarity and oriented cell divisions in the C. elegans embryo. Dev Cell 17:494–504
Muller A, Winkler J, Fiedler F, Sastradihardja T et al (2015) Oriented cell division in the C. elegans embryo is coordinated by G-protein signaling dependent on the adhesion GPCR LAT-1. PLoS Genet 11:e1005624
Langenhan T, Piao X, Monk KR (2016) Adhesion G protein-coupled receptors in nervous system development and disease. Nat Rev Neurosci 17:550–561
Nagar B, Overduin M, Ikura M, Rini JM (1996) Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature 380:360–364
Nishimura T, Honda H, Takeichi M (2012) Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell 149:1084–1097
Li S, Jin Z, Koirala S, Bu L et al (2008) GPR56 regulates pial basement membrane integrity and cortical lamination. J Neurosci 28:5817–5826
Chiang NY, Hsiao CC, Huang YS, Chen HY et al (2011) Disease-associated GPR56 mutations cause bilateral frontoparietal polymicrogyria via multiple mechanisms. J Biol Chem 286:14215–14225
Tissir F, Goffinet AM (2013) Shaping the nervous system: role of the core planar cell polarity genes. Nat Rev Neurosci 14:525–535
Ying G, Wu S, Hou R, Huang W et al (2009) The protocadherin gene Celsr3 is required for interneuron migration in the mouse forebrain. Mol Cell Biol 29:3045–3061
Qu Y, Huang Y, Feng J, Alvarez-Bolado G et al (2014) Genetic evidence that Celsr3 and Celsr2, together with Fzd3, regulate forebrain wiring in a Vangl-independent manner. Proc Natl Acad Sci U S A 111:E2996-3004
Ackerman SD, Garcia C, Piao X, Gutmann DH et al (2015) The adhesion GPCR Gpr56 regulates oligodendrocyte development via interactions with Galpha12/13 and RhoA. Nat Commun 6:6122
Monk KR, Naylor SG, Glenn TD, Mercurio S et al (2009) A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science 325:1402–1405
Monk KR, Oshima K, Jors S, Heller S et al (2011) Gpr126 is essential for peripheral nerve development and myelination in mammals. Development 138:2673–2680
Ackerman SD, Luo R, Poitelon Y, Mogha A et al (2018) GPR56/ADGRG1 regulates development and maintenance of peripheral myelin. J Exp Med 215:941–961
Mogha A, Benesh AE, Patra C, Engel FB et al (2013) Gpr126 functions in Schwann cells to control differentiation and myelination via G-protein activation. J Neurosci 33:17976–17985
Sigoillot SM, Iyer K, Binda F, Gonzalez-Calvo I et al (2015) The secreted protein C1QL1 and its receptor BAI3 control the synaptic connectivity of excitatory inputs converging on cerebellar Purkinje cells. Cell Rep 10:820–832
Kakegawa W, Mitakidis N, Miura E, Abe M et al (2015) Anterograde C1ql1 signaling is required in order to determine and maintain a single-winner climbing fiber in the mouse cerebellum. Neuron 85:316–329
Bottos A, Rissone A, Bussolino F, Arese M (2011) Neurexins and neuroligins: synapses look out of the nervous system. Cell Mol Life Sci 68:2655–2666
Kenzelmann D, Chiquet-Ehrismann R, Leachman NT, Tucker RP (2008) Teneurin-1 is expressed in interconnected regions of the developing brain and is processed in vivo. BMC Dev Biol 8:30
Lise MF, El-Husseini A (2006) The neuroligin and neurexin families: from structure to function at the synapse. Cell Mol Life Sci 63:1833–1849
Lu YC, Nazarko OV, Sando R 3rd, Salzman GS et al (2015) Structural basis of latrophilin-FLRT-UNC5 interaction in cell adhesion. Structure 23:1678–1691
Dark C, Homman-Ludiye J, Bryson-Richardson RJ (2018) The role of ADHD associated genes in neurodevelopment. Dev Biol 438:69–83
Lange M, Norton W, Coolen M, Chaminade M et al (2012) The ADHD-linked gene Lphn3.1 controls locomotor activity and impulsivity in zebrafish. Mol Psychiatry 17:855
Wallis D, Hill DS, Mendez IA, Abbott LC et al (2012) Initial characterization of mice null for Lphn3, a gene implicated in ADHD and addiction. Brain Res 1463:85–92
Piao X, Chang BS, Bodell A, Woods K et al (2005) Genotype-phenotype analysis of human frontoparietal polymicrogyria syndromes. Ann Neurol 58:680–687
Piao X, Hill RS, Bodell A, Chang BS et al (2004) G protein-coupled receptor-dependent development of human frontal cortex. Science 303:2033–2036
Piao X, Basel-Vanagaite L, Straussberg R, Grant PE et al (2002) An autosomal recessive form of bilateral frontoparietal polymicrogyria maps to chromosome 16q12.2-21. Am J Hum Genet 70:1028–1033
Lei Y, Zhu H, Yang W, Ross ME et al (2014) Identification of novel CELSR1 mutations in spina bifida. PLoS ONE 9:e92207
Qiao X, Liu Y, Li P, Chen Z et al (2016) Genetic analysis of rare coding mutations of CELSR1-3 in congenital heart and neural tube defects in Chinese people. Clin Sci (Lond) 130:2329–2340
Robinson A, Escuin S, Doudney K, Vekemans M et al (2012) Mutations in the planar cell polarity genes CELSR1 and SCRIB are associated with the severe neural tube defect craniorachischisis. Hum Mutat 33:440–447
Arcos-Burgos M, Jain M, Acosta MT, Shively S et al (2010) A common variant of the latrophilin 3 gene, LPHN3, confers susceptibility to ADHD and predicts effectiveness of stimulant medication. Mol Psychiatry 15:1053–1066
Labbe A, Liu A, Atherton J, Gizenko N et al (2012) Refining psychiatric phenotypes for response to treatment: contribution of LPHN3 in ADHD. Am J Med Genet B Neuropsychiatr Genet 159B:776–785
Choudhry Z, Sengupta SM, Grizenko N, Fortier ME et al (2012) LPHN3 and attention-deficit/hyperactivity disorder: interaction with maternal stress during pregnancy. J Child Psychol Psychiatry 53:892–902
Kappel DB, Schuch JB, Rovaris DL, da Silva BS et al (2019) ADGRL3 rs6551665 as a common vulnerability factor underlying attention-deficit/hyperactivity disorder and autism spectrum disorder. Neuromolecular Med 21:60–67
Huang X, Zhang Q, Gu X, Hou Y et al (2019) LPHN3 gene variations and susceptibility to ADHD in Chinese Han population: a two-stage case-control association study and gene-environment interactions. Eur Child Adolesc Psychiatry 28:861–873
Bonaglia MC, Marelli S, Novara F, Commodaro S et al (2010) Genotype-phenotype relationship in three cases with overlapping 19p13.12 microdeletions. Eur J Hum Genet 18:1302–1309
Willsey AJ, Fernandez TV, Yu D, King RA et al (2017) De novo coding variants are strongly associated with Tourette disorder. Neuron 94(486–499):e9
Wang S, Mandell JD, Kumar Y, Sun N et al (2018) De novo sequence and copy number variants are strongly associated with Tourette disorder and implicate cell polarity in pathogenesis. Cell Rep 24(3441–3454):e12
DeRosse P, Lencz T, Burdick KE, Siris SG et al (2008) The genetics of symptom-based phenotypes: toward a molecular classification of schizophrenia. Schizophr Bull 34:1047–1053
Purcell RH, Toro C, Gahl WA, Hall RA (2017) A disease-associated mutation in the adhesion GPCR BAI2 (ADGRB2) increases receptor signaling activity. Hum Mutat 38:1751–1760
Myers KA, Nasioulas S, Boys A, McMahon JM et al (2018) ADGRV1 is implicated in myoclonic epilepsy. Epilepsia 59:381–388
Weston MD, Luijendijk MW, Humphrey KD, Moller C et al (2004) Mutations in the VLGR1 gene implicate G-protein signaling in the pathogenesis of Usher syndrome type II. Am J Hum Genet 74:357–366
Patra C, Monk KR, Engel FB (2014) The multiple signaling modalities of adhesion G protein-coupled receptor GPR126 in development. Receptors Clin Investig 1:79
Patra C, van Amerongen MJ, Ghosh S, Ricciardi F et al (2013) Organ-specific function of adhesion G protein-coupled receptor GPR126 is domain-dependent. Proc Natl Acad Sci U S A 110:16898–16903
Waller-Evans H, Promel S, Langenhan T, Dixon J et al (2010) The orphan adhesion-GPCR GPR126 is required for embryonic development in the mouse. PLoS ONE 5:e14047
Torregrosa-Carrion R, Pineiro-Sabaris R, Siguero-Alvarez M, Grego-Bessa J et al (2021) Adhesion G protein-coupled receptor Gpr126/Adgrg6 is essential for placental development. Sci Adv 7:eabj5445
O'Donnell A, Yutzey KE (2020) Mechanisms of heart valve development and disease. Development 147
Doyle SE, Scholz MJ, Greer KA, Hubbard AD et al (2006) Latrophilin-2 is a novel component of the epithelial-mesenchymal transition within the atrioventricular canal of the embryonic chicken heart. Dev Dyn 235:3213–3221
Nechiporuk T, Urness LD, Keating MT (2001) ETL, a novel seven-transmembrane receptor that is developmentally regulated in the heart. ETL is a member of the secretin family and belongs to the epidermal growth factor-seven-transmembrane subfamily. J Biol Chem 276:4150–4157
Xiao J, Jiang H, Zhang R, Fan G et al (2012) Augmented cardiac hypertrophy in response to pressure overload in mice lacking ELTD1. PLoS ONE 7:e35779
Musa G, Engel FB, Niaudet C (2016) Heart development, angiogenesis, and blood-brain barrier function is modulated by adhesion GPCRs. Handb Exp Pharmacol 234:351–368
Scholz N, Gehring J, Guan C, Ljaschenko D et al (2015) The adhesion GPCR latrophilin/CIRL shapes mechanosensation. Cell Rep 11:866–874
Scholz N, Guan C, Nieberler M, Grotemeyer A et al (2017) Mechano-dependent signaling by latrophilin/CIRL quenches cAMP in proprioceptive neurons. Elife 6
Jakob Mitgau JF, Schinner C, Stephan G, Berndt S et al (2021) Mimicking extracellular matrix-mediated mechano-activation by antibodies to control signaling of the adhesion G protein-coupled receptor GPR126/ADGRG6. https://www.biorxiv.org/content/10.1101/2021.09.13.460127v1
Stephan G, Liebscher I, Placantonakis DG (2021) Activation of the adhesion GPCR GPR133 (ADGRD1) by antibodies targeting the N-terminus. https://www.biorxiv.org/content/10.1101/2021.09.13.460139v1.full
Cui H, Wang Y, Huang H, Yu W et al (2014) GPR126 protein regulates developmental and pathological angiogenesis through modulation of VEGFR2 receptor signaling. J Biol Chem 289:34871–34885
Stehlik C, Kroismayr R, Dorfleutner A, Binder BR et al (2004) VIGR–a novel inducible adhesion family G-protein coupled receptor in endothelial cells. FEBS Lett 569:149–155
Aird WC (2007) Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res 100:158–173
Paik DT, Tian L, Williams IM, Rhee S et al (2020) Single-cell RNA sequencing unveils unique transcriptomic signatures of organ-specific endothelial cells. Circulation 142:1848–1862
Nolan DJ, Ginsberg M, Israely E, Palikuqi B et al (2013) Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell 26:204–219
Lu S, Liu S, Wietelmann A, Kojonazarov B et al (2017) Developmental vascular remodeling defects and postnatal kidney failure in mice lacking Gpr116 (Adgrf5) and Eltd1 (Adgrl4). PLoS ONE 12:e0183166
Daneman R, Prat A (2015) The blood-brain barrier. Cold Spring Harb Perspect Biol 7:a020412
Cullen M, Elzarrad MK, Seaman S, Zudaire E et al (2011) GPR124, an orphan G protein-coupled receptor, is required for CNS-specific vascularization and establishment of the blood-brain barrier. Proc Natl Acad Sci U S A 108:5759–5764
Kuhnert F, Mancuso MR, Shamloo A, Wang HT et al (2010) Essential regulation of CNS angiogenesis by the orphan G protein-coupled receptor GPR124. Science 330:985–989
Yasukochi Y, Sakuma J, Takeuchi I, Kato K et al (2018) Six novel susceptibility loci for coronary artery disease and cerebral infarction identified by longitudinal exome-wide association studies in a Japanese population. Biomed Rep 9:123–134
Yamada Y, Kato K, Oguri M, Horibe H et al (2018) Identification of 13 novel susceptibility loci for early-onset myocardial infarction, hypertension, or chronic kidney disease. Int J Mol Med 42:2415–2436
Wu MP, Doyle JR, Barry B, Beauvais A et al (2013) G-protein coupled receptor 56 promotes myoblast fusion through serum response factor- and nuclear factor of activated T-cell-mediated signalling but is not essential for muscle development in vivo. FEBS J 280:6097–6113
White JP (2016) Control of skeletal muscle cell growth and size through adhesion GPCRs. Handb Exp Pharmacol 234:299–308
Hamoud N, Tran V, Croteau LP, Kania A et al (2014) G-protein coupled receptor BAI3 promotes myoblast fusion in vertebrates. Proc Natl Acad Sci U S A 111:3745–3750
Zyryanova T, Schneider R, Adams V, Sittig D et al (2014) Skeletal muscle expression of the adhesion-GPCR CD97: CD97 deletion induces an abnormal structure of the sarcoplasmatic reticulum but does not impair skeletal muscle function PLoS One 9:e100513
Karner CM, Long F, Solnica-Krezel L, Monk KR et al (2015) Gpr126/Adgrg6 deletion in cartilage models idiopathic scoliosis and pectus excavatum in mice. Hum Mol Genet 24:4365–4373
Wu S, Sun X, Zhu W, Huang Y et al (2012) Evidence for GAL3ST4 mutation as the potential cause of pectus excavatum. Cell Res 22:1712–1715
Kou I, Takahashi Y, Johnson TA, Takahashi A et al (2013) Genetic variants in GPR126 are associated with adolescent idiopathic scoliosis. Nat Genet 45:676–679
Khanshour AM, Kou I, Fan Y, Einarsdottir E et al (2018) Genome-wide meta-analysis and replication studies in multiple ethnicities identify novel adolescent idiopathic scoliosis susceptibility loci. Hum Mol Genet 27:3986–3998
Kou I, Watanabe K, Takahashi Y, Momozawa Y et al (2018) A multi-ethnic meta-analysis confirms the association of rs6570507 with adolescent idiopathic scoliosis. Sci Rep 8:11575
Qin X, Xu L, Xia C, Zhu W et al (2017) Genetic variant of GPR126 gene is functionally associated with adolescent idiopathic scoliosis in Chinese population. Spine (Phila Pa 1976) 42:E1098-E1103
Ravenscroft G, Nolent F, Rajagopalan S, Meireles AM et al (2015) Mutations of GPR126 are responsible for severe arthrogryposis multiplex congenita. Am J Hum Genet 96:955–961
Kitagaki J, Miyauchi S, Asano Y, Imai A et al (2016) A putative association of a single nucleotide polymorphism in GPR126 with aggressive periodontitis in a Japanese population. PLoS ONE 11:e0160765
Yates LL, Schnatwinkel C, Murdoch JN, Bogani D et al (2010) The PCP genes Celsr1 and Vangl2 are required for normal lung branching morphogenesis. Hum Mol Genet 19:2251–2267
Yates LL, Papakrivopoulou J, Long DA, Goggolidou P et al (2010) The planar cell polarity gene Vangl2 is required for mammalian kidney-branching morphogenesis and glomerular maturation. Hum Mol Genet 19:4663–4676
Brzoska HL, d’Esposito AM, Kolatsi-Joannou M, Patel V et al (2016) Planar cell polarity genes Celsr1 and Vangl2 are necessary for kidney growth, differentiation, and rostrocaudal patterning. Kidney Int 90:1274–1284
Wang XJ, Zhang DL, Xu ZG, Ma ML et al (2014) Understanding cadherin EGF LAG seven-pass G-type receptors. J Neurochem 131:699–711
Zaidman NA, Tomilin VN, Hassanzadeh Khayyat N, Damarla M et al (2020) Adhesion-GPCR Gpr116 (ADGRF5) expression inhibits renal acid secretion. Proc Natl Acad Sci U S A 117:26470–26481
Cazorla-Vazquez S, Engel FB (2018) Adhesion GPCRs in kidney development and disease. Front Cell Dev Biol 6:9
Pradervand S, Zuber Mercier A, Centeno G, Bonny O et al (2010) A comprehensive analysis of gene expression profiles in distal parts of the mouse renal tubule. Pflugers Arch 460:925–952
Harty BL, Krishnan A, Sanchez NE, Schioth HB et al (2015) Defining the gene repertoire and spatiotemporal expression profiles of adhesion G protein-coupled receptors in zebrafish. BMC Genomics 16:62
Qian YM, Haino M, Kelly K, Song WC (1999) Structural characterization of mouse CD97 and study of its specific interaction with the murine decay-accelerating factor (DAF, CD55). Immunology 98:303–311
Maiga A, Lemieux S, Pabst C, Lavallee VP et al (2016) Transcriptome analysis of G protein-coupled receptors in distinct genetic subgroups of acute myeloid leukemia: identification of potential disease-specific targets. Blood Cancer J 6:e431
Solaimani Kartalaei P, Yamada-Inagawa T, Vink CS, de Pater E et al (2015) Whole-transcriptome analysis of endothelial to hematopoietic stem cell transition reveals a requirement for Gpr56 in HSC generation. J Exp Med 212:93–106
Wang W, Jossin Y, Chai G, Lien WH et al (2016) Feedback regulation of apical progenitor fate by immature neurons through Wnt7-Celsr3-Fzd3 signalling. Nat Commun 7:10936
Posokhova E, Shukla A, Seaman S, Volate S et al (2015) GPR124 functions as a WNT7-specific coactivator of canonical beta-catenin signaling. Cell Rep 10:123–130
Li X, Roszko I, Sepich DS, Ni M et al (2013) Gpr125 modulates Dishevelled distribution and planar cell polarity signaling. Development 140:3028–3039
Scheller M, Huelsken J, Rosenbauer F, Taketo MM et al (2006) Hematopoietic stem cell and multilineage defects generated by constitutive beta-catenin activation. Nat Immunol 7:1037–1047
Reya T (2003) Regulation of hematopoietic stem cell self-renewal. Recent Prog Horm Res 58:283–295
Daga S, Rosenberger A, Quehenberger F, Krisper N et al (2019) High GPR56 surface expression correlates with a leukemic stem cell gene signature in CD34-positive AML. Cancer Med 8:1771–1778
Das S, Owen KA, Ly KT, Park D et al (2011) Brain angiogenesis inhibitor 1 (BAI1) is a pattern recognition receptor that mediates macrophage binding and engulfment of Gram-negative bacteria. Proc Natl Acad Sci U S A 108:2136–2141
Park D, Tosello-Trampont AC, Elliott MR, Lu M et al (2007) BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 450:430–434
Lee CS, Penberthy KK, Wheeler KM, Juncadella IJ et al (2016) Boosting apoptotic cell clearance by colonic epithelial cells attenuates inflammation in vivo. Immunity 44:807–820
Mazaheri F, Breus O, Durdu S, Haas P et al (2014) Distinct roles for BAI1 and TIM-4 in the engulfment of dying neurons by microglia. Nat Commun 5:4046
Wandel E, Saalbach A, Sittig D, Gebhardt C et al (2012) Thy-1 (CD90) is an interacting partner for CD97 on activated endothelial cells. J Immunol 188:1442–1450
Hamann J, Vogel B, van Schijndel GM, van Lier RA (1996) The seven-span transmembrane receptor CD97 has a cellular ligand (CD55, DAF). J Exp Med 184:1185–1189
Wang T, Ward Y, Tian L, Lake R et al (2005) CD97, an adhesion receptor on inflammatory cells, stimulates angiogenesis through binding integrin counterreceptors on endothelial cells. Blood 105:2836–2844
Kop EN, Kwakkenbos MJ, Teske GJ, Kraan MC et al (2005) Identification of the epidermal growth factor-TM7 receptor EMR2 and its ligand dermatan sulfate in rheumatoid synovial tissue. Arthritis Rheum 52:442–450
Hoek RM, de Launay D, Kop EN, Yilmaz-Elis AS et al (2010) Deletion of either CD55 or CD97 ameliorates arthritis in mouse models. Arthritis Rheum 62:1036–1042
Sutavani RV, Bradley RG, Ramage JM, Jackson AM et al (2013) CD55 costimulation induces differentiation of a discrete T regulatory type 1 cell population with a stable phenotype. J Immunol 191:5895–5903
Legrand F, Tomasevic N, Simakova O, Lee CC et al (2014) The eosinophil surface receptor epidermal growth factor-like module containing mucin-like hormone receptor 1 (EMR1): a novel therapeutic target for eosinophilic disorders. J Allergy Clin Immunol 133:1439–47, 1447 e1–8
Boyden SE, Desai A, Cruse G, Young ML et al (2016) Vibratory urticaria associated with a missense variant in ADGRE2. N Engl J Med 374:656–663
Iguchi T, Sakata K, Yoshizaki K, Tago K et al (2008) Orphan G protein-coupled receptor GPR56 regulates neural progenitor cell migration via a G alpha 12/13 and Rho pathway. J Biol Chem 283:14469–14478
Luo R, Jeong SJ, Jin Z, Strokes N et al (2011) G protein-coupled receptor 56 and collagen III, a receptor-ligand pair, regulates cortical development and lamination. Proc Natl Acad Sci U S A 108:12925–12930
Yeung J, Adili R, Stringham EN, Luo R et al (2020) GPR56/ADGRG1 is a platelet collagen-responsive GPCR and hemostatic sensor of shear force. Proc Natl Acad Sci U S A 117:28275–28286
Bridges JP, Ludwig MG, Mueller M, Kinzel B et al (2013) Orphan G protein-coupled receptor GPR116 regulates pulmonary surfactant pool size. Am J Respir Cell Mol Biol 49:348–357
Yang MY, Hilton MB, Seaman S, Haines DC et al (2013) Essential regulation of lung surfactant homeostasis by the orphan G protein-coupled receptor GPR116. Cell Rep 3:1457–1464
Haitina T, Olsson F, Stephansson O, Alsio J et al (2008) Expression profile of the entire family of adhesion G protein-coupled receptors in mouse and rat. BMC Neurosci 9:43
Ludwig MG, Seuwen K, Bridges JP (2016) Adhesion GPCR function in pulmonary development and disease. Handb Exp Pharmacol 234:309–327
Sakornsakolpat P, Prokopenko D, Lamontagne M, Reeve NF et al (2019) Genetic landscape of chronic obstructive pulmonary disease identifies heterogeneous cell-type and phenotype associations. Nat Genet 51:494–505
Hall RJ, O’Loughlin J, Billington CK, Thakker D et al (2021) Functional genomics of GPR126 in airway smooth muscle and bronchial epithelial cells. FASEB J 35:e21300
O’Hayre M, Vazquez-Prado J, Kufareva I, Stawiski EW et al (2013) The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat Rev Cancer 13:412–424
Kan Z, Jaiswal BS, Stinson J, Janakiraman V et al (2010) Diverse somatic mutation patterns and pathway alterations in human cancers. Nature 466:869–873
Aust G, Eichler W, Laue S, Lehmann I et al (1997) CD97: a dedifferentiation marker in human thyroid carcinomas. Cancer Res 57:1798–1806
Zendman AJ, Cornelissen IM, Weidle UH, Ruiter DJ et al (1999) TM7XN1, a novel human EGF-TM7-like cDNA, detected with mRNA differential display using human melanoma cell lines with different metastatic potential. FEBS Lett 446:292–298
Nallanthighal S, Heiserman JP, Cheon DJ (2019) The role of the extracellular matrix in cancer stemness. Front Cell Dev Biol 7:86
Kaur B, Brat DJ, Devi NS, Van Meir EG (2005) Vasculostatin, a proteolytic fragment of brain angiogenesis inhibitor 1, is an antiangiogenic and antitumorigenic factor. Oncogene 24:3632–3642
Kaur B, Cork SM, Sandberg EM, Devi NS et al (2009) Vasculostatin inhibits intracranial glioma growth and negatively regulates in vivo angiogenesis through a CD36-dependent mechanism. Cancer Res 69:1212–1220
Aust G, Zhu D, Van Meir EG, Xu L (2016) Adhesion GPCRs in tumorigenesis. Handb Exp Pharmacol 234:369–396
Safaee M, Clark AJ, Oh MC, Ivan ME et al (2013) Overexpression of CD97 confers an invasive phenotype in glioblastoma cells and is associated with decreased survival of glioblastoma patients. PLoS ONE 8:e62765
Li J, Shen J, Wang Z, Xu H et al (2019) ELTD1 facilitates glioma proliferation, migration and invasion by activating JAK/STAT3/HIF-1alpha signaling axis. Sci Rep 9:13904
Izutsu T, Konda R, Sugimura J, Iwasaki K et al (2011) Brain-specific angiogenesis inhibitor 1 is a putative factor for inhibition of neovascular formation in renal cell carcinoma. J Urol 185:2353–2358
Inamura K (2017) Renal cell tumors: understanding their molecular pathological epidemiology and the 2016 WHO Classification. Int J Mol Sci 18
Low G, Huang G, Fu W, Moloo Z et al (2016) Review of renal cell carcinoma and its common subtypes in radiology. World J Radiol 8:484–500
Shingarev R, Jaimes EA (2017) Renal cell carcinoma: new insights and challenges for a clinician scientist. Am J Physiol Renal Physiol 313:F145–F154
Richter GH, Fasan A, Hauer K, Grunewald TG et al (2013) G-protein coupled receptor 64 promotes invasiveness and metastasis in Ewing sarcomas through PGF and MMP1. J Pathol 230:70–81
Masiero M, Simoes FC, Han HD, Snell C et al (2013) A core human primary tumor angiogenesis signature identifies the endothelial orphan receptor ELTD1 as a key regulator of angiogenesis. Cancer Cell 24:229–241
Hatanaka H, Oshika Y, Abe Y, Yoshida Y et al (2000) Vascularization is decreased in pulmonary adenocarcinoma expressing brain-specific angiogenesis inhibitor 1 (BAI1). Int J Mol Med 5:181–183
Bari MF, Brown H, Nicholson AG, Kerr KM et al (2014) BAI3, CDX2 and VIL1: a panel of three antibodies to distinguish small cell from large cell neuroendocrine lung carcinomas. Histopathology 64:547–556
Olaniru OE, Persaud SJ (2019) Adhesion G-protein coupled receptors: Implications for metabolic function. Pharmacol Ther 198:123–134
Cortijo C, Gouzi M, Tissir F, Grapin-Botton A (2012) Planar cell polarity controls pancreatic beta cell differentiation and glucose homeostasis. Cell Rep 2:1593–1606
Korpos E, Kadri N, Kappelhoff R, Wegner J et al (2013) The peri-islet basement membrane, a barrier to infiltrating leukocytes in type 1 diabetes in mouse and human. Diabetes 62:531–542
Blodgett DM, Nowosielska A, Afik S, Pechhold S et al (2015) Novel observations from next-generation RNA sequencing of highly purified human adult and fetal islet cell subsets. Diabetes 64:3172–3181
Huang Y, Fan J, Yang J, Zhu GZ (2008) Characterization of GPR56 protein and its suppressed expression in human pancreatic cancer cells. Mol Cell Biochem 308:133–139
Olaniru OE, Pingitore A, Giera S, Piao X et al (2018) The adhesion receptor GPR56 is activated by extracellular matrix collagen III to improve beta-cell function. Cell Mol Life Sci 75:4007–4019
Duner P, Al-Amily IM, Soni A, Asplund O et al (2016) Adhesion G protein-coupled receptor G1 (ADGRG1/GPR56) and pancreatic beta-cell function. J Clin Endocrinol Metab 101:4637–4645
Rothe J, Thor D, Winkler J, Knierim AB et al (2019) Involvement of the adhesion GPCRs latrophilins in the regulation of insulin release. Cell Rep 26(1573–1584):e5
Gupta R, Nguyen DC, Schaid MD, Lei X et al (2018) Complement 1q-like-3 protein inhibits insulin secretion from pancreatic beta-cells via the cell adhesion G protein-coupled receptor BAI3. J Biol Chem 293
Suchy T, Zieschang C, Popkova Y, Kaczmarek I et al (2020) The repertoire of adhesion G protein-coupled receptors in adipocytes and their functional relevance. Int J Obes (Lond) 44:2124–2136
Nie T, Hui X, Gao X, Li K et al (2012) Adipose tissue deletion of Gpr116 impairs insulin sensitivity through modulation of adipose function. FEBS Lett 586:3618–3625
Georgiadi A, Lopez-Salazar V, Merahbi RE, Karikari RA et al (2021) Orphan GPR116 mediates the insulin sensitizing effects of the hepatokine FNDC4 in adipose tissue. Nat Commun 12:2999
Bradley EC, Cunningham RL, Wilde C, Morgan RK et al (2019) In vivo identification of small molecules mediating Gpr126/Adgrg6 signaling during Schwann cell development. Ann N Y Acad Sci 1456:44–63
Gupte J, Swaminath G, Danao J, Tian H et al (2012) Signaling property study of adhesion G-protein-coupled receptors. FEBS Lett 586:1214–1219
Southern C, Cook JM, Neetoo-Isseljee Z, Taylor DL et al (2013) Screening beta-arrestin recruitment for the identification of natural ligands for orphan G-protein-coupled receptors. J Biomol Screen 18:599–609
Stoveken HM, Larsen SD, Smrcka AV, Tall GG (2018) Gedunin- and khivorin-derivatives are small-molecule partial agonists for adhesion G protein-coupled receptors GPR56/ADGRG1 and GPR114/ADGRG5. Mol Pharmacol 93:477–488
Stoveken HM, Bahr LL, Anders MW, Wojtovich AP et al (2016) Dihydromunduletone is a small-molecule selective adhesion G protein-coupled receptor antagonist. Mol Pharmacol 90:214–224
Demberg LM, Winkler J, Wilde C, Simon KU et al (2017) Activation of adhesion G protein-coupled receptors: agonist specificity of stachel sequence-derived peptides. J Biol Chem 292:4383–4394
Sun Y, Zhang D, Ma ML, Lin H et al (2021) Optimization of a peptide ligand for the adhesion GPCR ADGRG2 provides a potent tool to explore receptor biology. J Biol Chem 296:100174
Demberg LM, Rothemund S, Schoneberg T, Liebscher I (2015) Identification of the tethered peptide agonist of the adhesion G protein-coupled receptor GPR64/ADGRG2. Biochem Biophys Res Commun 464:743–747
Barros-Alvarez X, Nwokonko RM, Vizurraga A, Matzov D et al (2022) The tethered peptide activation mechanism of adhesion GPCRs. Nature 604:757–762
Ping YQ, Xiao P, Yang F, Zhao RJ et al (2022) Structural basis for the tethered peptide activation of adhesion GPCRs. Nature 604:763–770
Qu X, Qiu N, Wang M, Zhang B et al (2022) Structural basis of tethered agonism of the adhesion GPCRs ADGRD1 and ADGRF1. Nature 604:779–785
Xiao P, Guo S, Wen X, He QT et al (2022) Tethered peptide activation mechanism of the adhesion GPCRs ADGRG2 and ADGRG4. Nature 604:771–778
Jo M, Jung ST (2016) Engineering therapeutic antibodies targeting G-protein-coupled receptors. Exp Mol Med 48:e207
Yona S, Lin HH, Dri P, Davies JQ et al (2008) Ligation of the adhesion-GPCR EMR2 regulates human neutrophil function. FASEB J 22:741–751
Lizano E, Hayes JL, Willard FS (2021) A synthetic method to assay adhesion-family G-protein coupled receptors. Determination of the G-protein coupling profile of ADGRG6(GPR126). Biochem Biophys Res Commun 534:317–322
Mathiasen S, Palmisano T, Perry NA, Stoveken HM et al (2020) G12/13 is activated by acute tethered agonist exposure in the adhesion GPCR ADGRL3. Nat Chem Biol 16:1343–1350
Funding
This work was supported by a Ramalingaswamy fellowship from the Department of Biotechnology, India and intramural funding from Ashoka University to KP.
Author information
Authors and Affiliations
Contributions
AS, MT, and KP wrote the manuscript. KP prepared figures and edited the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Content in this manuscript did not require ethics approval as there were no animals or human subjects involved in the study.
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sreepada, A., Tiwari, M. & Pal, K. Adhesion G protein-coupled receptor gluing action guides tissue development and disease. J Mol Med 100, 1355–1372 (2022). https://doi.org/10.1007/s00109-022-02240-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-022-02240-0