Abstract
Key message
Gene distributions and population genomics suggest artificial selection of ghd7 osprr37, for extremely early heading date of rice, in the Tohoku region of Japan.
Abstract
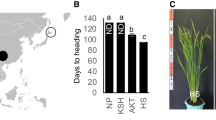
The ranges of cultivated crops expanded into various environmental conditions around the world after their domestication. Hokkaido, Japan, lies at the northern limit of cultivation of rice, which originated in the tropics. Novel genotypes for extremely early heading date in Hokkaido are controlled by loss-of-function of both Grain number, plant height and heading date 7 (Ghd7) and Oryza sativa Pseudo-Response Regulator 37 (OsPRR37). We traced genotypes for extremely early heading date and analyzed the phylogeny of rice varieties grown historically in Japan. The mutations in Ghd7 and OsPRR37 had distinct local distributions. Population genomics revealed that varieties collected from the Tohoku region of northern Japan formed three clusters. Mutant alleles of Ghd7 and OsPRR37 appear to have allowed rice cultivation to spread into Hokkaido. Our results show that the mutations of two genes might be occurred in the process of artificial selection during early rice cultivation in the Tohoku region.







Similar content being viewed by others
References
Agrama HA, Yan WG, Jia M, Fjellstrom R, McClung AM (2010) Genetic structure associated with diversity and geographic distribution in the USDA rice world collection. Nat Sci 2:247–291
Alexander DH, Novembre J, Lange K (2009) Fast model-based estimation of ancestry in unrelated individuals. Genome Res 19:1655–1664
Browning BL, Zhou Y, Browning SR (2018) A one-penny imputed genome from next-generation reference panels. Am J Hum Genet 103:338–348
Chang CC et al (2015) Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4:7
Choi JY, Platts AE, Fuller DQ, Hsing YI, Wing RA, Purugganan MD (2017) The rice paradox: multiple origins but single domestication in Asian rice. Mol Biol Evol 34:969–979
Danecek P et al (2011) The variant call format and VCFtools. Bioinformatics 27:2156–2158
Ebana K, Kojima Y, Fukuoka S, Nagamine T, Kawase M (2008) Development of mini core collection of Japanese rice landrace. Breed Sci 58:281–291
Fujino K (2020) Days to heading, controlled by the heading date genes, Hd1 and DTH8, limits rice yield-related traits in Hokkaido. Jpn Breed Sci 70(3):277–282
Fujino K, Ikegaya T (2020) A novel genotype DATTO5 developed using the five genes exhibits the fastest heading date designed in rice. Breed Sci 70(2):193–199
Fujino K, Sekiguchi H (2005a) Mapping of QTLs conferring extremely early heading in rice (Oryza sativa L.). Theor Appl Genet 111:393–398
Fujino K, Sekiguchi H (2005b) Identification of QTLs conferring genetic variation for heading date among rice varieties at the northern-limit of rice cultivation. Breed Sci 55:141–146
Fujino K, Yamanouchi U (2020) Genetic effect of a new allele for the flowering time locus Ghd7 in rice. Breed Sci 70(3):342–346
Fujino K, Sekiguchi H, Sato T, Kiuchi H, Nonoue Y, Takeuchi Y, Ando T, Lin SY, Yano M (2004) Mapping of quantitative trait loci controlling low-temperature germinability in rice (Oryza sativa L.). Theor Appl Genet 108:794–799
Fujino K, Sekiguchi H, Kiguchi T (2005) Identification of an active transposon in intact rice plants. Mol Genet Genom 273:150–157
Fujino K, Yamanouchi U, Yano M (2013) Roles of the Hd5 gene controlling heading date for adaptation to the northern limits of rice cultivation. Theor Appl Genet 126:611–618
Fujino K, Obara M, Ikegaya T, Tamura K (2015) Genetic shift in local rice populations during rice breeding programs in the northern limit of rice cultivation in the world. Theor Appl Genet 128:1739–1746
Fujino K, Nishimura T, Kiuchi H, Hirayama Y, Sato T (2017) Phenotypic changes during 100-year rice breeding programs in Hokkaido. Breed Sci 67:528–534
Fujino K, Hirayama Y, Obara M, Ikegaya T (2018) Colocalization of QTLs for hull-cracked rice and grain size in elite rice varieties in Japan. Breed Sci 68:449–454
Fujino K, Yamanouchi U, Nonoue Y, Obara M, Yano M (2019a) Switching genetic effects of the flowering time gene Hd1 under LD conditions by Ghd7 and OsPRR37 in rice. Breed Sci 69:127–132
Fujino K, Obara M, Ikegaya T (2019b) Establishment of adaptability to the northern-limit of rice production. Mol Genet Genom 294(3):729–737
Fujino K, Hirayama Y, Kaji R (2019c) Marker-assisted selection in rice breeding programs in Hokkaido. Breed Sci 69(3):383–392
Fuller DQ (2011) Pathways to Asian civilizations: tracing the origins and spread of rice and rice cultures. Rice 4:78–92
Gao H, Jin M, Zheng XM, Chen J, Yuan D, Xin Y, Wang M, Huang D, Zhang Z, Zhou K et al (2014) Days to heading 7, a major quantitative locus determining photoperiod sensitivity and regional adaptation in rice. Proc Natl Acad Sci USA 111:16337–16342
Guo T, Mu Q, Wang J, Vanous AE, Onogi A, Iwata H, Li X, Yu J (2020) Dynamic effects of interacting genes underlying rice flowering-time phenotypic plasticity and global adaptation. Genome Res 30(5):673–683
Han Z, Zhang B, Zhao H, Ayaad M, Xing Y (2016) Genome-wide association studies reveal that diverse heading date genes respond to short and long day lengths between indica and japonica rice. Front Plant Sci 7:1270
Haudry A, Cenci A, Ravel C, Bataillon T, Brunel D, Poncet C, Hochu I, Poirier S, Santoni S, Glemin S, David J (2007) Grinding up wheat: a massive loss of nucleotide diversity since domestication. Mol Biol Evol 24(7):1506–1517
Hu Y, Li S, Xing Y (2019) Lessons from natural variations: artificially induced heading date variations for improvement of regional adaptation in rice. Theor Appl Genet 132(2):383–394
Huang X, Kurata N, Wei X, Wang ZX, Wang A, Zhao Q, Zhao Y, Liu K, Lu H, Li W et al (2012) A map of rice genome variation reveals the origin of cultivated rice. Nature 490:497–501
Hyten DL, Song Q, Zhu Y, Choi IY, Nelson RL, Costa JM, Specht JE, Shoemaker RC, Cregan PB (2006) Impacts of genetic bottlenecks on soybean genome diversity. Proc Natl Acad Sci USA 103:16666–16671
International Rice Genome Sequencing Project (2005) The map-based sequence of the rice genome. Nature 436:793–800
Koo BH, Yoo SC, Park JW, Kwon CT, Lee BD, An G, Zhang Z, Li J, Li Z, Paek NC (2013) Natural variation in OsPRR37 regulates heading date and contributes to rice cultivation at a wide range of latitudes. Mol Plant 6:1877–1888
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25(14):1754–1760
Li X, Liu H, Wang M, Liu H, Tian X, Zhou W, Lu T, Wang Z, Chu C, Fang J, Bu Q (2015) Combinations of Hd2 and Hd4 genes determine rice adaptability to Heilongjiang Province, northern limit of China. J Integr Plant Biol 57:698–707
Li W, Liu L, Wang Y, Zhang Q, Fan G, Zhang S, Wang Y, Liao K (2020) Genetic diversity, population structure, and relationships of apricot (Prunus) based on restriction site-associated DNA sequencing. Hortic Res 7:69
Lin H, Liang ZW, Sasaki T, Yano M (2003) Fine mapping and characterization of quantitative trait loci Hd4 and Hd5 controlling heading date in rice. Breed Sci 53:51–59
Lu JJ, Chang TT (1980) Rice in its temporal and spatial perspectives. In: Luh BS (ed) Rice: production and utilization. AVI Publishing Co., Inc, Westport, CT, pp 1–74
Morales-Hojas R, Sun J, Iraizoz FA, Tan X, Chen J (2020) Contrasting population structure and demographic history of cereal aphids in different environmental and agricultural landscapes. Ecol Evol 10(18):9647–9662
Murakami M, Ashikari M, Miura K, Yamashino T, Mizuno T (2003) The evolutionarily conserved OsPRR quintet: rice pseudo-response regulators implicated in circadian rhythm. Plant Cell Physiol 44(11):1229–1236
Murakami M, Tago Y, Yamashino T, Mizuno T (2007) Comparative overviews of clock-associated genes of Arabidopsis thaliana and Oryza sativa. Plant Cell Physiol 48:110–121
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucl Acid Res 8:4321–4325
Nakamichi N, Kita M, Ito S, Yamashino T, Mizuno T (2005) Pseudo-response regulators, PRR9, PRR7 and PRR5, together play essential roles close to the circadian clock of Arabidopsis thaliana. Plant Cell Physiol 46:686–698
Nonoue Y, Fujino K, Hirayama Y, Yamanouchi U, Lin SY, Yano M (2008) Detection of quantitative trait loci controlling extremely early heading in rice. Theor Appl Genet 116:715–722
Peterson BK, Weber JN, Kay EH, Fisher HS, Hoekstra HE (2012) Double digest RADseq: an inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PLoS ONE 7(5):e37135
Sansaloni C, Franco J, Santos B, Percival-Alwyn L, Singh S, Petroli C, Campos J, Dreher K, Payne T, Marshall D et al (2020) Diversity analysis of 80 000 wheat accessions reveals consequences and opportunities of selection footprints. Nat Commun 11(1):4572
Shibaya T, Nonoue Y, Ono N, Yamanouchi U, Hori K, Yano M (2011) Genetic interactions involved in the inhibition of heading date QTL, Hd2 in rice under long-day conditions. Theor Appl Genet 123:1133–1143
Shinada H, Yamamoto T, Yamamoto E, Hori K, Yonemaru J, Matsuba S, Fujino K (2014) Historical changes in population structure during rice breeding programs in the northern limits of rice cultivation. Theor Appl Genet 127:995–1004
Shirasawa K, Hirakawa H, Isobe S (2016) Analytical workflow of double-digest restriction site-associated DNA sequencing based on empirical and in silico optimization in tomato. DNA Res 23(2):145–153
Sun J, Ma D, Tang L, Zhao M, Zhang G et al (2019) Population genomic analysis and de novo assembly reveal the origin of weedy rice as an evolutionary game. Mol Plant 12(5):632–647
Tanaka N, Shenton M, Kawahara Y, Kumagai M, Sakai H, Kanamori H, Yonemaru J, Fukuoka S, Sugimoto K, Ishimoto M et al (2021) Investigation of the genetic diversity of a core collection of Japanese rice landraces (JRC) using whole-genome sequencing. Plant Cell Physiol 61(12):2087–2096
Tenaillon MI, U’Ren J, Tenaillon O, Gaut BS (2004) Selection versus demography: a multilocus investigation of the domestication process in maize. Mol Biol Evol 21:1214–1225
van der Auwera AG, Carneiro OM, Hartl C, Poplin R, Del Angel G, Levy-Moonshine A, Jordan T, Shakir K, Roazen D, Thibault J et al (2013) From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr Protoc Bioinform 43:11.10.1-11.10.33
Xue W, Xing Y, Weng X, Zhao Y, Tang W, Wang L, Zhou H, Yu S, Xu C, Li X, Zhang Q (2008) Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet 40:761–767
Yamamoto T, Lin H, Sasaki T, Yano M (2000) Identification of heading date quantitative trait locus Hd6 and characterization of its epistatic interactions with Hd2 in rice using advanced backcross progeny. Genetics 154(2):885–891
Yano M, Katayose Y, Ashikari M, Yamanouchi U, Monna L, Fuse T, Baba T, Yamamoto K, Umehara Y, Nagamura Y, Sasaki T (2000) Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 12:2473–2483
Zhang J, Zhou X, Yan W, Zhang Z, Lu L, Han Z, Zhao H, Liu H, Song P, Hu Y et al (2015) Combinations of the Ghd7, Ghd8 and Hd1 genes largely define the ecogeographical adaptation and yield potential of cultivated rice. New Phytol 208:1056–1066
Zhang B, Liu H, Qi F, Zhang Z, Li Q, Han Z, Xing Y (2019) Genetic interactions among Ghd7, Ghd8, OsPRR37 and Hd1 contribute to large variation in heading date in rice. Rice 12:48
Zhao K, Tung CW, Eizenga GC et al (2011) Genome-wide association mapping reveals a rich genetic architecture of complex traits in Oryza sativa. Nat Commun 2:467
Zhenhua Z, Yujun Z, Shilin W, Yeyang F, Jieyun Z (2021) Genetic interaction of Hd1 with Ghd7, DTH8 and Hd2 largely determine the eco-geographical adaption of rice varieties in southern China. Rice Sci 28(2):114–118
Acknowledgements
We thank M. Obara (NARO) for assistance with DNA experiments. The wild rice accessions were supplied by the National Institute of Genetics, supported by the National Bioresource Project (NBRP), AMED, Japan. This work was supported in part by a grant from the Iijima Memorial Foundation for the Promotion of Food Science and Technology (to KF) and by the Advanced Analysis Center Research Supporting Program of NARO.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments and wrote the manuscript: KF. Performed the experiments, analyzed the data, and approved the final manuscript: KF, YK, KS.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Additional information
Communicated by Lixi Jiang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fujino, K., Kawahara, Y. & Shirasawa, K. Artificial selection in the expansion of rice cultivation. Theor Appl Genet 135, 291–299 (2022). https://doi.org/10.1007/s00122-021-03966-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-021-03966-0