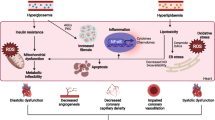
Abstract
Aims/hypothesis
N6-methyladenosine (m6A) mRNA methylation and m6A-related proteins (methyltransferase-like 3 [METTL3], methyltransferase-like 14 [METTL14] and YTH domain containing 1 [YTHDC1]) have been shown to regulate islet beta cell function and the pathogenesis of diabetes. However, whether Wilms’ tumour 1-associating protein (WTAP), a key regulator of the m6A RNA methyltransferase complex, regulates islet beta cell failure during pathogenesis of diabetes is largely unknown. The present study aimed to investigate the role of WTAP in the regulation of islet beta cell failure and diabetes.
Methods
Islet beta cell-specific Wtap-knockout and beta cell-specific Mettl3-overexpressing mice were generated for this study. Blood glucose, glucose tolerance, serum insulin, glucose-stimulated insulin secretion (both in vivo and in vitro), insulin levels, glucagon levels and beta cell apoptosis were examined. RNA-seq and MeRIP-seq were performed, and the data were well analysed.
Results
WTAP was downregulated in islet beta cells in type 2 diabetes, due to lipotoxicity and chronic inflammation, and islet beta cell-specific deletion of Wtap (Wtap-betaKO) induced beta cell failure and diabetes. Wtap-betaKO mice showed severe hyperglycaemia (above 20 mmol/l [360 mg/dl]) from 8 weeks of age onwards. Mechanistically, WTAP deficiency decreased m6A mRNA modification and reduced the expression of islet beta cell-specific transcription factors and insulin secretion-related genes by reducing METTL3 protein levels. Islet beta cell-specific overexpression of Mettl3 partially reversed the abnormalities observed in Wtap-betaKO mice.
Conclusions/interpretation
WTAP plays a key role in maintaining beta cell function by regulating m6A mRNA modification depending on METTL3, and the downregulation of WTAP leads to beta cell failure and diabetes.
Data availability
The RNA-seq and MeRIP-seq datasets generated during the current study are available in the Gene Expression Omnibus database repository (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE215156; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE215360).
Graphical abstract

Similar content being viewed by others

Introduction
Islet beta cell failure is the major cause of diabetes. Islet beta cell-specific transcriptional factors such as MAF bZIP transcription factor A (MafA), NK6 homeobox 1 (NKX6-1), pancreatic and duodenal homeobox 1 (PDX1), neuronal differentiation 1 (NEUROD1) and forkhead box A2 (FOXA2) play an important role in maintaining beta cell function [1,2,3,4,5,6,7]. These transcriptional factors control the expression of key insulin secretion-related genes including Ins1, Ins2, Gck and Glut2 [1,2,3,4,5,6,7]. Mutations in any of these transcriptional factors and key insulin secretion-related genes cause islet beta cell failure and diabetes [1,2,3,4,5,6,7]. Some of the genes belong to the MODY family, including GCK (MODY2), PDX1 (MODY4), NEUROD1 (MODY6), INS (MODY10) [8]. An abundance of data shows that these transcription factors and key insulin secretion-related genes are regulated at the transcriptional level [1,2,3,4,5,6,7]. Whether and how they undergo post-transcriptional regulation by RNA processing are not fully understood. Some evidence shows that RNA processing such as N6-methyladenosine (m6A) mRNA modification regulates islet beta cell function [9,10,11]. However, the detailed molecular mechanisms are not fully understood.
m6A mRNA methylation is catalysed by m6A methyltransferase complex. Methyltransferase-like 3 (METTL3) is the key m6A methyltransferase, and Wilms’ tumour 1-associating protein (WTAP) interacts with METTL3 and methyltransferase-like 14 (METTL14) in the nucleus, serving as a regulatory protein of m6A mRNA modification [12]. m6A mRNA methylation can be recognised by its reader proteins (YTH domain containing 1/2 [YTHDC1/2] and YTH N6-methyladenosine RNA binding protein 1–3 [YTHDF1–3]) [13, 14]. m6A mRNA methylation is reversible and can be demethylated by its eraser proteins (FTO α-ketoglutarate dependent dioxygenase [FTO] and AlkB homolog 5, RNA demethylase [ALKBH5]) [13, 14]. Previous work by our group and others shows that m6A-related proteins are involved in regulating obesity [15], non-alcoholic steatohepatitis (NASH) [16, 17] and diabetes [9,10,11, 18, 19]. For example, METTL3 and WTAP play a key role in the postnatal development of interscapular brown adipose tissue (iBAT), which is associated with energy metabolism and obesity [15, 20]. We have also shown that WTAP regulates postnatal development of iBAT by stabilising METTL3 [20] and that both METTL3 and WTAP negatively regulate the pathogenesis of NASH [16, 17]. METTL3 and METTL14 are required for the neonatal maturation of islet beta cells [18]. m6A modification, Mettl3, Mettl14 and Ythdc1 are significantly downregulated in the islets of humans with type 2 diabetes, and islet beta cell-specific deletion of either Mettl3, Mettl14 or Ythdc1 leads to hyperglycaemia due to islet beta cell failure [9,10,11, 18, 19]. These previous studies have demonstrated that m6A mRNA methylation and its related proteins (METTL3, METTL14 and YTHDC1) play key roles in maintaining islet beta cell function, and their downregulation during the pathogenesis of diabetes leads to beta cell failure and diabetes. However, whether WTAP regulates beta cell failure and diabetes is largely unknown, and whether WTAP regulates islet beta cell function depending on METTL3 is also unknown. Therefore, building on our previous work and that of others, we investigated these two important questions in this study.
Methods
Animal experiments
Animal experiments were approved by the Institutional Animal Care and Use Committee of Harbin Institute of Technology (HIT/IACUC). Mice were housed under controlled light (12h light–dark cycle), temperature (24±2°C) and humidity (50±10%) conditions and fed a normal chow diet with a free access to water. Wtapflox/flox mice, in which the exon 4 of the Wtap gene was flanked by two loxp sites, were generated using the CRISPR-Cas9 technique as described previously [17, 20]. Wtapflox/flox mice were crossed with C57BL/6J mice expressing Cre under the rat insulin-2 promoter (RIP-Cre mice) [21] to generate islet beta cell-specific Wtap-knockout (Wtap-betaKO) mice. The genotype of Wtap-betaKO mice was Wtapflox/floxRIP-Cre+/−. Wtapflox/flox mice served as a control. STOP-Mettl3 mice were generated using the CRISPR-Cas9 technique to insert a STOP-FLAG-Mettl3 cassette into the Rosa26 allele as described previously [16, 20]. To generate islet beta cell-specific Mettl3-overexpressing and Wtap-knockout (Wtap-betaKO/Mettl3-betaOE) mice, STOP-Mettl3 mice were crossed with Wtap-betaKO mice. The genotype of the Wtap-betaKO/Mettl3-betaOE mice is Wtapflox/floxSTOP-Mettl3+/−RIP-Cre+/−. The genotype of the Mettl3-betaOE mice is STOP-Mettl3+/−RIP-Cre+/−. STOP-Mettl3+/−mice were used as a control for Mettl3-betaOE mice. Wtapflox/flox, STOP-Mettl3+/−and RIP-Cre+/− mice were on the C57BL/6J background. Blood glucose levels were measured as described previously [9]. Blood samples were collected from the orbital sinus. Determination of glucose-stimulated insulin secretion (GSIS) in vivo is described in electronic supplementary material (ESM) Methods. Serum glucagon and insulin levels were measured using glucagon ELISA kits (DGCG0; R&D Systems; USA) and insulin ELISA kits (MS100; EZassay, China), respectively. The sex, age, genotype and number of mice are described in the relevant figure legends.
Cell culture
INS-1 832/13 cells (RRID: CVCL_7226) were cultured at 37°C and 5% CO2 in RPMI-1640 medium supplemented with 10% FBS and 50 mmol/l β-mercaptoethanol as described previously [22, 23]. INS-1 832/13 cells were treated with cytokines (TNF-α 20 ng/ml, IL-β 20 ng/ml and IFN-γ 200 ng/ml) or palmitic acid (PA) (0.5 mmol/l) overnight and subjected to quantitative reverse transcription PCR (qRT-PCR) and immunoblotting assays.
Pancreatic islet isolation
Pancreases from male mice were harvested, cut into small pieces and incubated at 37°C for 10 min in Hanks’ balanced salt solution (HBSS) (pH 7.4) supplemented with 5 mmol/l glucose and 1 mg/ml collagenase P (Roche Diagnostics, Germany). Individual islets were hand-picked and cultured at 37°C and 5% CO2 in RPMI-1640 medium supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin. Determination of GSIS in vitro, and RNA-seq and methylated RNA immunoprecipitation sequencing (MeRIP-seq), were carried out as described in ESM Methods. RNA-seq and MeRIP-seq data that support the findings of this study have been deposited in the Gene Expression Omnibus (GEO) under accession codes GSE215156 and GSE215360.
Immunoprecipitation and immunoblotting
INS-1 832/13 cells were infected with Ad-betaGal, Ad-FLAG-METTL3 or Ad-FLAG-WTAP adenovirus overnight. Total cell lysates were harvested in lysis buffer (R0020; Solarbio, China), immunoprecipitated with anti-FLAG antibody (F1804; Sigma, USA) or IgG1 control, and then immunoblotted with anti-WTAP or anti-METTL3 antibodies. Antibody dilution ratios were as follows: METTL3 (96391; CST, USA; 1:2500 dilution; RRID: AB_2800261); FLAG (F1804; Sigma, USA; 1:5000 dilution; RRID: AB_262044); FLAG (20543-1-AP; Proteintech, China; 1:5000 dilution); β-actin (60008-1-lg; Proteintech, China; 1:5000); and WTAP (10200-1-lg; Proteintech; 1:2500 dilution). The primary antibodies were diluted in PBST containing 3% wt/vol. BSA. Goat anti-mouse IgG (H+L), HRP (ZB-2305; ZSGB-BIO, China; 1:5000 dilution) and Goat anti-rabbit IgG (H+L) secondary antibody, HRP (ZB-2301; ZSGB-BIO; 1:5000 dilution) were diluted in PBST containing 5% wt/vol. milk.
Immunostaining and TUNEL assays
Pancreases were fixed in 4% wt/vol. paraformaldehyde for 3 h and then in 30% wt/vol. sucrose overnight. Five frozen pancreatic sections (10 μm, spaced >200 μm apart) were taken from each mouse for staining and analysis. These sections were stained with the indicated antibodies. The dilution ratios were as follows: insulin (A0564; Dako, Denmark; 1:1000 dilution); glucagon (G2654; Sigma; 1: 500 dilution; RRID: AB_259852); FLAG (F1804; Sigma; 1:500 dilution). Goat anti-guinea pig IgG (H+L)/AF488 antibody (bs-0358G-AF488; Bioss, China; 1:1000 dilution) and cy3 goat anti-mouse IgG (H+L) antibody (K1207; APExBIO, China; 1:1000 dilution) were secondary antibodies. Primary and secondary antibodies were diluted in PBS containing 5% vol./vol. goat serum and 1% wt/vol. BSA. Islet insulin- and glucagon-positive areas were measured using Image J software version 1.39f (National Institutes of Health, USA) and normalised to pancreatic section areas. TUNEL assays were performed by using cell death detection kits (Roche Diagnostics). The sections were also stained with DAPI to visualise total cells.
Statistical analysis
Data are presented as means ±SEM. Differences between two groups were analysed by two-tailed Student’s t tests. Differences between three groups were analysed by one-factor ANOVA and the least significance difference t test. In all analyses, p<0.05 was considered statistically significant. The Kolmogorov–Smirnov normality test with p>0.1 suggested our samples followed a normal distribution. Statistical analyses and figures were made using GraphPad Prism version 6.02 (GraphPad Software, USA). ANOVA and least significance difference t test analyses were performed using SPSS 21.0 (SPSS, USA).
Results
WTAP is downregulated in diabetic islets
It has been shown that METTL3, METTL14 and YTHDC1 are required for islet beta cell function [9, 18, 19], and their expression is downregulated in islets from individuals with type 2 diabetes [10, 18, 19]. WTAP interacts with METTL3 and METTL14 to form an m6A methyltransferase complex [12]. WTAP serves as a regulatory protein [12]. We investigated whether WTAP also contributes to the pathogenesis of beta cell failure and diabetes. We analysed the published islet single-cell RNA-seq data (GSE153855) from individuals with and without type 2 diabetes [24]. As shown in Fig. 1a, the reads per kilobase per million mapped reads (RPKM) values of Wtap were significantly decreased in type 2 diabetes. Lipotoxicity, chronic inflammation and hyperglycaemia contribute to the pathogenesis of type 2 diabetes [25]. It has been shown that METTL3 is downregulated by chronic inflammation and oxidative stress in islet beta cells [9]. Mettl3 was also downregulated by lipotoxicity (PA treatment) in INS-1 832/13 cells (ESM Fig. 1a). We asked whether WTAP is regulated by lipotoxicity, chronic inflammation and hyperglycaemia. As shown in Fig. 1b–e, Wtap mRNA was downregulated by treatment with PA or cytokines (TNF-α, IL-1β and IFN-γ) in both primary islets and INS-1 832/13 cells. Cytokines decreased Wtap mRNA levels in a time dependent manner (Fig. 1e). Consistently, WTAP protein was also downregulated by treatment with PA or cytokines in both primary islets and INS-1 832/13 cells (Fig. 1f–i). However, high glucose treatment (25 mmol/l) did not change the expression of WTAP (ESM Fig. 1b). These data indicate that WTAP is downregulated in islet beta cells of type 2 diabetes likely due to lipotoxicity and chronic inflammation.
WTAP is downregulated in diabetic islets. (a) Wtap RPKM values in single beta cells from a high-quality pancreatic islet single-cell RNA-seq (scRNA-seq) dataset from individuals with and without type 2 diabetes (GSE153855). (b, c, f, g) Pancreatic islets were isolated from C57BL/6 mice (100 islets from one or two mice in each sample). These samples underwent an overnight treatment with or without PA (0.5 mmol/l). PA (0.5 mmol/l) was also applied to INS-1 832/13 cells overnight. qRT-PCR was used to determine Wtap mRNA levels (b, c) (n=4 or 5). Relative Wtap mRNA levels were normalised to 36B4. Immunoblotting analysis was used to measure WTAP and actin protein levels (f) and Image J was used to quantify those levels (g) (n=3 independent samples for islets; n=3 separate samples for INS-1 832/13 cells). Relative WTAP protein levels were normalised to actin. (d, e, h, i) One hundred pancreatic islets from one or two mice were incubated in each sample. Islet samples were treated with cytokines (TNF-α 20 ng/ml, IL-1β 20 ng/ml and IFN-γ 200 ng/ml) or not for 2 h. Cytokine treatments were also applied to INS-1 832/13 cells for different times (0, 1, 2, 4 and 6 h). qRT-PCR was used to measure Wtap mRNA levels (d, e) (n=4 or 5). Relative Wtap mRNA levels were normalised to 36B4. For immunoblotting, INS-1 832/13 cells were treated with or without cytokines overnight. Immunoblotting analysis was used to measure the WTAP and actin protein levels (h), and Image J was used to quantify those levels (i) (n=3 independent samples for islets; n=3 separate samples for INS-1 832/13 cells). Relative WTAP protein levels were normalised to actin. *p<0.05, **p<0.01 and ***p<0.001. Data represent the mean ± SEM. Con, control (no treatment); Cyto, cytokines; T2D, type 2 diabetes
Islet beta cell-specific deletion of Wtap causes diabetes
To test whether Wtap downregulation in pancreatic beta cells contributes to diabetes, we created Wtap-betaKO mice by crossing Wtapflox/flox mice with RIP-Cre transgenic mice. RIP-Cre+/− and Wtapflox/flox mice showed similar body weight and blood glucose (ESM Fig. 2a, b). Therefore, we used Wtapflox/flox mice as the control for Wtap-betaKO mice. WTAP protein levels were reduced by 62% in the islets of Wtap-betaKO mice vs Wtapflox/flox mice, as expected (Fig. 2a,b). Both male and female Wtap-betaKO mice had body weights comparable with Wtap flox/flox mice (ESM Fig. 3a, b). However, starting at 4 weeks of age, feeding blood glucose levels in male Wtap-betaKO mice gradually increased, reaching approximately 20 mmol/l (360 mg/dl) at 8 weeks of age and remaining above 20 mmol/l after 8 weeks of age, whereas at 3–12 weeks of age, blood glucose levels of male Wtapflox/flox control mice remained constant (Fig. 2c). The fasting blood glucose levels in male Wtap-betaKO mice were also significantly higher than those in male RIP-Cre+/−and Wtapflox/flox mice (ESM Fig. 2b). Consistently, female Wtap-betaKO mice also showed hyperglycaemia (Fig. 2d). Furthermore, Wtap-betaKO mice demonstrated severe glucose intolerance in both male and female animals (Fig. 2e–h). The glucose AUCs were considerably elevated (by 2.7- and 2.46-fold, respectively) in male and female Wtap-betaKO mice (Fig. 2f,h). Male and female Wtap-betaKO mice both displayed insulin sensitivity at 8 weeks of age comparable with that shown in Wtapflox/flox mice (ESM Fig. 3c–f). These data indicate that both male and female Wtap-betaKO mice displayed hyperglycaemia and glucose intolerance. Therefore, we used male mice for subsequent experiments. Male Wtap-betaKO mice at 12 weeks of age had an 87% reduction in serum insulin levels (Fig. 2i), indicating dramatically decreased insulin secretion. Consistently, at 8 weeks of age, male Wtap-betaKO mice had severely reduced GSIS (Fig. 2j). We also noted that the basal (fasting) serum insulin levels in male Wtap-betaKO mice at 8 weeks of age were also dramatically reduced, by 67.3% (Fig. 2j). GSIS and insulin content were also dramatically reduced in islets isolated from male Wtap-betaKO mice at 7 weeks of age (Fig. 2k,l). Consistently, male Wtap-betaKO mice at 12 weeks of age showed a 97.2% decrease in pancreatic insulin content (Fig. 2m). These data demonstrate that beta cell-specific deletion Wtap leads to beta cell failure and diabetes due to severely impaired insulin secretion.
Islet beta cell-specific deletion of Wtap leads to beta cell failure and diabetes. (a, b) Pancreatic islets were isolated from 7-week-old male Wtap-betaKO and Wtapflox/flox mice (100 islets from 4–6 Wtap-betaKO mice or 2 or 3 Wtapflox/flox mice were present in each sample). Immunoblotting analysis was used to determine the levels of WTAP and actin proteins (a), and Image J was used for quantification (b) (n=3 independent samples). Relative WTAP protein levels were normalised to actin. (c) From 3 to 12 weeks of age, male Wtap-betaKO and Wtapflox/flox mice had their feeding blood glucose levels monitored (Wtap-betaKO, n=9 or 10; Wtapflox/flox, n=10). (d) At 8 weeks of age, female Wtap-betaKO and Wtapflox/flox mice had their feeding blood glucose levels measured (Wtap-betaKO, n=5; Wtapflox/flox, n=5). (e, f) At 8 weeks of age, male Wtap-betaKO and Wtapflox/flox mice underwent GTTs (Wtap-betaKO, n=8; Wtapflox/flox, n=8). Blood glucose levels (e) and AUC (f) were determined. (g, h) At 8 weeks of age, female Wtap-betaKO and Wtapflox/flox mice underwent GTTs (Wtap-betaKO, n=7; Wtapflox/flox, n=8). Blood glucose levels (g) and AUC (h) were determined. (i) At 12 weeks of age, male Wtap-betaKO and Wtapflox/flox mice had their serum insulin levels measured (Wtap-betaKO, n=8; Wtapflox/flox, n=8). (j) At 8 weeks of age, male Wtap-betaKO and Wtapflox/flox mice had their GSIS tested (Wtap-betaKO, n=8; Wtapflox/flox, n=8). (k, l) At 7 weeks of age, isolated islets from male Wtap-betaKO and Wtapflox/flox mice were examined for GSIS (basal glucose concentration 2.8 mmol/l; high glucose concentration 16.7 mmol/l) and insulin content (Wtap-betaKO, n=5; Wtapflox/flox, n=5). (m) At 12 weeks of age, male Wtap-betaKO and Wtapflox/flox mice had their pancreas insulin content measured (Wtap-betaKO, n=8; Wtapflox/flox, n=8). *p<0.05, **p<0.01 and ***p<0.001. Data represent the mean ± SEM. n is the number of biologically independent mice
Islet beta cell-specific deletion of Wtap reduces insulin-positive area
Reduced pancreatic insulin content may be associated with decreased insulin-positive area. To test this possibility, immunofluorescence analysis was performed. As shown in Fig. 3a,b, the relative insulin-positive area in Wtap-betaKO mice at 12 weeks of age was dramatically decreased, by 97.3%, when compared with control Wtapflox/flox mice. Serum glucagon levels, pancreatic glucagon content and the relative glucagon-positive area were similar when comparing male Wtapflox/flox and Wtap-betaKO mice at 12 weeks of age (ESM Fig. 3g–i), indicating that glucagon is less likely to contribute to hyperglycaemia in Wtap-betaKO mice. To determine whether beta cell death contributed to the observed reduction in insulin-positive area in Wtap-betaKO mice, we measured islet beta cell apoptosis by TUNEL staining. Pancreatic sections were co-immunostained with an anti-insulin antibody to visualise beta cells. The number of TUNEL-positive beta cells was much greater in Wtap-betaKO islets than in their littermate control (Fig. 3c,d). These data suggest that beta cell-specific Wtap deletion leads to beta cell death, causing a significant reduction in insulin-positive area and glucose tolerance in Wtap-betaKO mice.
Islet beta cell-specific deletion of Wtap leads to reduced insulin-positive area due to increased beta cell death. (a, b) At 12 weeks of age, pancreatic sections from male Wtap-betaKO and Wtapflox/flox mice were immunostained for insulin and glucagon. Representative islet images are shown (a). Scale bar, 100 μm. The amount of insulin-positive area per pancreatic section was measured (Wtap-betaKO, n=5; Wtapflox/flox, n=5) (b). (c, d) TUNEL assays were used to measure the apoptosis of islet beta cells in male Wtap-betaKO and Wtapflox/flox mice at 8 weeks of age (Wtap-betaKO, n=6; Wtapflox/flox, n=6). Representative islet images are shown (c). Scale bar, 100 μm. TUNEL-positive cells were counted and normalised to total cell number (d). *p<0.05 and ***p<0.001. Data represent the mean ± SEM. n is the number of biologically independent mice
Islet beta cell-specific deletion of Wtap changes gene expression profile in islets
To further determine the molecular mechanisms by which WTAP regulates beta cell function, we performed RNA-seq analysis in islets isolated from male Wtap-betaKO and Wtapflox/flox mice. As shown in Fig. 4a, 2900 genes were upregulated and 3015 genes were downregulated in islets from Wtap-betaKO mice. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis showed that protein processing in endoplasmic reticulum (ER), synaptic vesicle cycle, protein export, insulin secretion, tricarboxylic acid (TCA) cycle, type 2 diabetes, MODY, insulin signalling pathway, mammalian target of rapamycin (mTOR) signalling pathway and prolactin signalling pathways were downregulated (Fig. 4b), whereas ribosome, extracellular matrix (ECM)-receptor interaction, cell adhesion molecules, and antigen processing and presentation were upregulated (ESM Fig. 4a). Gene ontology (GO) analysis indicated that the downregulated genes were primarily related to Golgi vesicle transport, vesicle organisation, response to ER stress, ER organisation, cellular response to hormone stimulus, response to insulin, and Golgi organisation (Fig. 4c), whereas the upregulated genes were related to collagen metabolic process, angiogenesis, extracellular structure organisation, cell-substrate adhesion, and response to wounding (ESM Fig. 4b). Among the differentially regulated genes, we noticed that apoptosis-related genes (including Bcl10, Casp6, Casp7, Cd24a, Dedd2, Dnase2a, Rps3 and Xkr8) were upregulated, whereas anti-apoptosis-related genes (including Acvr1c, Akt1, Cdk5rap3, and Madd) were downregulated in Wtap-betaKO mouse islets (ESM Fig. 4c). This increase in apoptosis-related genes and decrease in anti-apoptosis-related genes may contribute to the increased apoptosis in islet beta cells of Wtap-betaKO.
Islet beta cell-specific deletion of Wtap decreases expression of beta cell-specific transcription factors and insulin secretion-related genes. (a) At 7 weeks of age, primary islets were extracted from male Wtap-betaKO and Wtapflox/flox mice and subjected to RNA-seq analysis (n=3 independent mice). A volcano plot was used to display the differentially expressed genes (DEGs) (Wtap-betaKO vs Wtapflox/flox), which included 3015 downregulated genes and 2900 upregulated genes (|log2[foldchange]| >0, p<0.05). (b) Analysis of the downregulated genes’ KEGG signalling pathways. (c) The enriched GO biological process terms for the genes that were downregulated are displayed. (d) A Venn diagram of the RNA-seq data from Wtap-betaKO vs Wtapflox/flox and Mettl3-betaKO vs Mettl3flox/flox islets revealed that 1131 genes were downregulated in both Wtap-betaKO and Mettl3-betaKO islets. These 1131 downregulated genes were further analysed by KEGG and GO enrichment analysis, respectively. (e) Heatmap results revealed that in Wtap-betaKO islets, the beta cell-specific transcription factors and insulin secretion-related genes were downregulated
WTAP, METTL3 and METTL14 form a m6A methyltransferase complex [12]. METTL3 serves as a key m6A methyltransferase and WTAP is a regulatory protein [12]. Both Wtap-betaKO and beta cell-specific Mettl3-knockout (Mettl3-betaKO) mice showed beta cell failure and diabetes. It is necessary to determine whether WTAP and METTL3 regulate the same set of genes. Combined analysis of RNA-seq data from Wtap-betaKO vs Wtapflox/flox and Mettl3-betaKO vs Mettl3flox/flox mouse islets showed that 1131 genes were downregulated in these two datasets (Fig. 4d). KEGG pathway enrichment analysis showed that insulin secretion, protein processing in ER, MODY, type 2 diabetes, and insulin signalling pathways were downregulated (Fig. 4d). GO analysis indicated that the downregulated genes were primarily related to signal release, Golgi vesicle transport, peptide hormone secretion, hormone secretion, and insulin secretion (Fig. 4d). The downregulated genes included those encoding key transcriptional factors (e.g. Mafa, Nkx6-1, Pdx1, Neurod1 and Foxa2) in islet beta cells, and other important insulin secretion-related genes (e.g. Ins1, Ins2, Brsk2, Cacna1c, Doc2b, Ffar1, G6pc2, Gck, Gipr, Hadh, Ica1, Nnat, Park7, Pclo, Selenot, Serp1, Slc30a8, Stxbp51, Sytl4, Trpm2, Ucn3 and Uqcc2) (Fig. 4e). These results suggest that both WTAP and METTL3 are required for maintaining the expression of beta cell-specific transcription factors and insulin secretion-related genes.
WTAP is necessary for m6A mRNA modification of insulin secretion-related transcripts
The molecular mechanisms by which WTAP controls islet beta cell function were then investigated. It has been demonstrated that WTAP interacts with METTL3 in the nucleus and acts as a METTL3 regulatory protein [12, 20, 26]. We recently reported that METTL3 controls RNA m6A modification, which is necessary for islet beta cell function [9]. It is possible that WTAP regulates islet beta cell function through METTL3. To test this hypothesis, we initially verified that WTAP interacted with METTL3 in beta cells (Fig. 5a–d). WTAP may control the stability of the METTL3 protein, as shown by the reduced METTL3 protein but not mRNA levels in Wtap-betaKO mouse islets (Fig. 5e,f). Interestingly, BAT-specific deletion of Wtap also resulted in the reduction of METTL3 protein levels, through decreasing the stability of METTL3 protein depending on proteasome [20]. These results indicate that WTAP is essential for maintaining METTL3 protein stability.
Islet beta cell-specific deletion of Wtap leads to beta cell failure due to decreased m6A modification and expression of insulin secretion-related genes. (a–d) INS-1 832/13 cells were infected with Ad-betaGal, Ad-FLAG-METTL3 or Ad-FLAG-WTAP adenovirus overnight. Total cell lysates were immunoprecipitated (IP) with FLAG beads or IgG1 control. Anti-WTAP or anti-METTL3 antibodies were then used for immunoblotting (IB). Representative immunoblotting images are shown (a, c). The levels of METTL3 co-IP with FLAG-WTAP or IgG1 were normalised to the input METTL3 (b) and the levels of WTAP co-IP with FLAG-METTL3 or IgG1 were normalised to the input WTAP (d). n=3 independent results in three independent experiments, with similar results. (e) Immunoblotting analysis was used to measure the amounts of METTL3 and actin proteins in islets isolated from male Wtapflox/flox and Wtap-betaKO mice at 7 weeks old (n=3). (f) Heatmap of RNA-seq data showing the relative Mettl3 mRNA levels in islets of male Wtapflox/flox and Wtap-betaKO mice at 7 weeks old (n=3). (g) At 7 weeks of age, primary islets from male Wtap-betaKO and Wtapflox/flox mice were subjected to MeRIP-seq analysis. A volcano plot depicts the differential peaks (|log2[foldchange]| >1, p<0.05). (h) The downregulated genes in Wtap-betaKO islets were analysed by KEGG pathway enrichment analysis. (i) Venn diagram illustrating 354 transcripts with decreased m6A peaks in both MeRIP-seq datasets from Wtap-betaKO vs Wtapflox/flox and Mettl3-betaKO vs Mettl3flox/flox mouse islets. (j) These 354 genes were further analysed by GO enrichment analysis. **p<0.01. Data represent the mean ± SEM. n is the number of biologically independent cell samples or mice
We carried out an m6A RNA immunoprecipitation sequencing (MeRIP-seq) study in islets of Wtap-betaKO and Wtapflox/flox mice in order to further ascertain whether m6A modification of mRNA regulated by WTAP was connected to islet beta cell function. The m6A peaks identified in islets of Wtapflox/flox mice were enriched at the stop codon and 3′-UTR and were characterised by the canonical GGACU motif (ESM Fig. 5a, b), which is consistent with previously published MeRIP-seq results [9, 20, 27]. Although the m6A peaks identified in islets of Wtap-betaKO mice were also enriched at stop codon and 3′-UTR (ESM Fig. 5c), no GGACU motif was characterised. We found around 10,964 significant m6A peaks (false discovery rate <0.05) in roughly 6250 transcripts in the islets of Wtapflox/flox mice (ESM Table 1). There was a total of 2057 differential peaks, including 1503 downregulated and 554 upregulated m6A peaks in the islets of Wtap-betaKO mice (Fig. 5g and ESM Table 2). The genes with downregulated m6A peaks included beta cell-specific transcription factors and insulin secretion-related genes (Fig. 5g and ESM Table 2), which may contribute to the reduction of their mRNA levels. According to KEGG analysis, genes with downregulated m6A peaks were related to protein processing in ER, MODY, MAPK signalling, regulation of actin cytoskeleton, ubiquitin-mediated proteolysis, focal adhesion, axon guidance, and PI3K–Akt signalling pathways (Fig. 5h). METTL3 serves as a key m6A methyltransferase, and WTAP is a regulatory protein [12]. Both Wtap-betaKO and Mettl3-betaKO mice showed beta cell failure and diabetes. It is necessary to determine whether WTAP and METTL3 regulate m6A modification of the same set of genes. Combined analysis of MeRIP-seq data from Wtap-betaKO vs Wtapflox/flox and Mettl3-betaKO vs Mettl3flox/flox mouse islets showed 354 transcripts exhibiting decreased m6A levels in these two datasets (Fig. 5i). GO analysis showed that transcripts exhibiting decreased m6A levels were associated with regulation of hormone secretion, hormone transport, regulation of insulin secretion, regulation of peptide hormone secretion, and signal release (Fig. 5j). These data suggest that both WTAP and METTL3 regulate m6A modification of insulin secretion-related transcripts.
Islet beta cell-specific overexpression of Mettl3 partially reverses beta cell failure and diabetes in Wtap-betaKO mice
Islet beta cell-specific deletion of Wtap resulted in impaired beta cell function, which is likely due to the decreased METTL3-mediated RNA m6A modification. To determine whether METTL3 is really involved in this process, we restored METTL3 expression in islet beta cells of Wtap-betaKO mice by crossing islet Mettl3-betaOE with Wtap-betaKO mice. As shown in Fig. 6a, FLAG-tagged METTL3 was overexpressed in islet beta cells of Wtap-betaKO/Mettl3-betaOE but not Wtap-betaKO or Wtapflox/flox mice. Under normal conditions, Mettl3-betaOE and STOP-Mettl3 mice showed similar body weight and blood glucose (ESM Fig. 6a, b). However, islet beta cell-specific overexpression of Mettl3 in Wtap-betaKO mice partially reversed beta cell failure and diabetes in the Wtap-betaKO mice, as revealed by decreased hyperglycaemia (Fig. 6b), without changing body weight (Fig. 6c); further findings included improved glucose tolerance (Fig. 6d,e) (without changes in insulin sensitivity; Fig. 6f,g), increased plasma insulin levels (Fig. 6h), elevated insulin content (Fig. 6i) and increased insulin-positive area (Fig. 6j,k). Glucagon-positive areas (Fig. 6j,l) were similar in these three genotypes of mice. These data indicate that WTAP regulates beta cell function partially depending on METTL3.
Islet beta cell-specific overexpression of METTL3 partially reverses beta cell failure and diabetes in Wtap-betaKO mice. STOP-FLAG-Mettl3 transgenic mice were crossed with Wtap-betaKO mice to generate Wtap-betaKO/Mettl3-betaOE mice. (a) Co-immunostaining of insulin and FLAG-METTL3 with anti-insulin and anti-FLAG antibodies in pancreatic sections of male Wtapflox/flox, Wtap-betaKO and Wtap-betaKO/Mettl3-betaOE mice at 8 weeks old. Representative images are shown. Scale bar, 100 μm. (b, c) Feeding blood glucose levels (b) and body weight (c) were measured in male Wtapflox/flox, Wtap-betaKO and Wtap-betaKO/Mettl3-betaOE mice from 3 to 7 weeks of age (n=6 for each group). (d–g) GTTs (d) and ITTs (f) were performed in male Wtapflox/flox, Wtap-betaKO and Wtap-betaKO/Mettl3-betaOE mice at 7 weeks of age (n=6 for each group); AUC for GTTs and relative AUC for ITTs are shown (e, g). (h, i) Serum insulin levels and pancreas insulin content were measured in male Wtapflox/flox, Wtap-betaKO and Wtap-betaKO/Mettl3-betaOE mice at 7 weeks of age (n=8 for each group). (j–l) Pancreas sections of male Wtapflox/flox, Wtap-betaKO and Wtap-betaKO/Mettl3-betaOE mice at 8 weeks of age were immunostained for insulin and glucagon. Representative images are shown (j). Scale bar, 100 μm. Insulin-positive (k) and glucagon-positive (l) areas per pancreatic section were quantified (n=5 for each group). §§p<0.01 and §§§p<0.001, Wtap-betaKO vs Wtapflox/flox mice; *p<0.05, **p<0.01 and ***p<0.001, Wtap-betaKO/Mettl3-betaOE vs Wtap-betaKO mice. Data represent the mean ± SEM. n is the number of biologically independent mice
Discussion
Maintenance of islet beta cell function is required for controlling blood glucose homeostasis. Impaired islet beta cell function leads to hyperglycaemia and diabetes. Elucidating the molecular mechanisms of islet beta cell dysfunction or failure is very important to find novel treatments for diabetes. Some evidence shows that m6A mRNA modification regulates beta cell function [9,10,11]. In this study, we demonstrated that WTAP is downregulated in islets in type 2 diabetes by lipotoxicity and chronic inflammation, and that beta cell-specific deletion of Wtap leads to beta cell failure and diabetes likely due to decreased m6A modification and expression of insulin secretion-related transcripts.
Our previous research and other studies have demonstrated that m6A modification, its key writer proteins (METTL3/METTL14), and its reader protein YTHDC1 are significantly downregulated in the islets of humans with type 2 diabetes likely due to lipotoxicity, oxidative stress and chronic inflammation [9,10,11, 18, 19]. In this study, we show that WTAP, another key m6A writer protein, is also downregulated in the islet beta cells of humans with type 2 diabetes likely due to lipotoxicity and chronic inflammation. Multiple signalling pathways may contribute to the downregulation of m6A signalling pathway in islets during type 2 diabetes. For example, our previous studies have demonstrated that activation of the non-canonical NF-κB signalling pathway leads to the downregulation of METTL3 and YTHDC1 [9, 19], and overexpression of NF-κB-inducing kinase (NIK) induces beta cell failure and diabetes [28]. These results indicate that NIK activation causes the downregulation of m6A-related proteins and further leads to beta cell failure and diabetes. However, we cannot rule out other possible molecular mechanisms that also contribute to the downregulation of m6A-related proteins in islets during type 2 diabetes. Because the access to human pancreatic islets is limited, most of the regulation assays were performed in mouse islets or INS-1 832/13 cells. Developing good human beta cell lines will solve this problem.
Downregulation of m6A and its related proteins in islet beta cells results in beta cell failure and diabetes [9,10,11, 18, 19]. Islet beta cell-specific deletion of Mettl3, Mettl14, Ythdc1 or Wtap leads to hyperglycaemia and diabetes due to impaired insulin secretion at a young age [9,10,11, 18, 19]. One possible explanation is that m6A and its related proteins are required for the postnatal maturation of islet beta cells [18]. Another tissue-specific knockout mouse model supports this explanation. BAT-specific deletion of either Mettl3 or Wtap impairs the postnatal development of iBAT [15, 20].
Wtap-betaKO mice show very similar phenotypes to the phenotypes observed in Mettl3-betaKO mice [9]. Both mice show hyperglycaemia, hypoinsulinaemia and diabetes, with a dramatically reduced insulin-positive islet area and impaired insulin secretion. Significantly decreased expression of beta cell-specific transcription factors and insulin secretion-related genes results in these abnormalities observed in both Wtap-betaKO and Mettl3-betaKO mice. However, it should be noticed that loss of beta cells in these two knockout mice would naturally reduce the abundance of beta cell-specific genes. The reduction in insulin-positive area may be attributed to beta cell apoptosis. Some apoptosis-related genes are upregulated, whereas some anti-apoptosis-related genes are downregulated in Wtap-betaKO mouse islets. m6A peaks in Cd24a and Dedd2 transcripts are differentially regulated in Wtap-betaKO mouse islets. Whether WTAP directly regulates these apoptosis- and anti-apoptosis-related transcripts depending on m6A modification is still unknown. Meanwhile, both mRNA levels and m6A peaks of Pdx1 were significantly decreased in Wtap-betaKO mouse islets. Downregulation of Pdx1 may also contribute to beta cell apoptosis in Wtap-betaKO mouse islets because it has been reported that half deletion of Pdx1 induces beta cell apoptosis and diabetes [29].
Wtap deficiency decreases METTL3 protein levels but does not affect Mettl3 mRNA levels in islets, and BAT-specific deletion of Wtap shows similar results in iBAT [20]. WTAP binds to METTL3 in many cell types including beta cells and iBAT. WTAP is essential for maintaining the protein stability of METTL3 [20]. Many of the transcripts that show a decrease in m6A modification when Wtap is specifically deleted from islet beta cells are consistent with those seen in Mettl3-betaKO mouse islets. The m6A modifications in many transcripts encoding beta cell-specific transcription factors and insulin secretion-related proteins are decreased in both Wtap-betaKO and Mettl3-betaKO islets. It is likely that YTHDC1 identifies these m6A modifications and controls the expression of beta cell-specific transcription factors and insulin secretion-related genes [19]. In Wtap-betaKO mice, overexpression of METTL3 in islet beta cells partially reverses the hyperglycaemia/diabetes/hypoinsulinaemia. These findings suggest that WTAP controls beta cell function via METTL3. We discovered that overexpressing METTL3 just in islet beta cells does not completely reverse the abnormalities seen in Wtap-betaKO mice, suggesting that other molecular processes may also be involved in WTAP function in islet beta cells. Our recent research demonstrates that WTAP and METTL3 can bind to gene promoters and control chromatin accessibility [16, 17]. It is important to find out whether WTAP and METTL3 can control gene transcription directly in islet beta cells.
WTAP and METTL3, as m6A writer proteins, are essential for maintaining islet beta cell function [9]. YTHDC1, as an m6A reader protein, plays a key role in beta cell failure [19]. It is necessary to determine whether m6A eraser proteins (FTO and ALKBH5) promote islet beta cell failure and diabetes in vivo. Maintaining m6A levels or homeostasis might be a good strategy for the treatment of islet beta cell failure and diabetes.
In conclusion, we have demonstrated that WTAP is downregulated in islets in type 2 diabetes by lipotoxicity and chronic inflammation, and that beta cell-specific deletion of Wtap leads to beta cell failure and diabetes due to reduced METTL3-mediated m6A modification and decreased expression of beta cell-specific key transcription factors and insulin secretion-related genes. Islet beta cell-specific overexpression of Mettl3 partially reverses the abnormalities observed in Wtap-betaKO mice. These data suggest that WTAP plays an essential role in maintaining beta cell function partially by stabilising METTL3, and also indicate that downregulation of either WTAP or METTL3 contributes to beta cell failure during the pathogenesis of diabetes.
Data availability
The RNA-seq and MeRIP-seq datasets generated during the current study are available in the Gene Expression Omnibus database repository (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE215156; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE215360).
Abbreviations
- BAT:
-
Brown adipose tissue
- iBAT:
-
Interscapular brown adipose tissue
- ER:
-
Endoplasmic reticulum
- GO:
-
Gene Ontology
- GSIS:
-
Glucose-stimulated insulin secretion
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- m6A:
-
N6-methyladenosine
- MeRIP-seq:
-
Methylated RNA immunoprecipitation sequencing
- METTL3:
-
Methyltransferase-like 3
- METTL14:
-
Methyltransferase-like 14
- Mettl3-betaKO:
-
Beta cell-specific Mettl3-knockout
- Mettl3-betaOE:
-
Beta cell-specific Mettl3-overexpressing
- NASH:
-
Non-alcoholic steatohepatitis
- NIK:
-
NF-κB-inducing kinase
- PA:
-
Palmitic acid
- qRT-PCR:
-
Quantitative reverse transcription PCR
- RPKM:
-
Reads per kilobase per million mapped reads
- WTAP:
-
Wilms’ tumour 1-associating protein
- Wtap-betaKO:
-
Beta cell-specific Wtap-knockout
- YTHDC1:
-
YTH domain containing 1
References
Zhang C, Moriguchi T, Kajihara M et al (2005) MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol 25:4969–4976. https://doi.org/10.1128/MCB.25.12.4969-4976.2005
Nishimura W, Takahashi S, Yasuda K (2015) MafA is critical for maintenance of the mature beta cell phenotype in mice. Diabetologia 58:566–574. https://doi.org/10.1007/s00125-014-3464-9
Schaffer AE, Taylor BL, Benthuysen JR et al (2013) Nkx6.1 controls a gene regulatory network required for establishing and maintaining pancreatic Beta cell identity. PLoS Genet 9:e1003274–e1003274. https://doi.org/10.1371/journal.pgen.1003274
Li Y, Cao X, Li LX, Brubaker PL, Edlund H, Drucker DJ (2005) β-Cell Pdx1 expression is essential for the glucoregulatory, proliferative, and cytoprotective actions of glucagon-like peptide-1. Diabetes 54:482–491. https://doi.org/10.2337/diabetes.54.2.482
Brissova M, Shiota M, Nicholson WE et al (2002) Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J Biol Chem 277:11225–11232. https://doi.org/10.1074/jbc.M111272200
Gu C, Stein GH, Pan N et al (2010) Pancreatic beta cells require NeuroD to achieve and maintain functional maturity. Cell Metab 11:298–310. https://doi.org/10.1016/j.cmet.2010.03.006
Gao N, White P, Doliba N, Golson ML, Matschinsky FM, Kaestner KH (2007) Foxa2 Controls vesicle docking and insulin secretion in mature β cells. Cell Metab 6:267–279. https://doi.org/10.1016/j.cmet.2007.08.015
Naylor R, Knight Johnson A, del Gaudio D (1993) Maturity-onset diabetes of the young overview. University of Washington, Seattle, Seattle (WA)
Li X, Jiang Y, Sun X, Wu Y, Chen Z (2021) METTL3 is required for maintaining β-cell function. Metab Clin Exp 116:154702. https://doi.org/10.1016/j.metabol.2021.154702
De Jesus DF, Zhang Z, Kahraman S et al (2019) m(6)A mRNA methylation regulates human β-cell biology in physiological states and in type 2 diabetes. Nat Metab 1:765–774. https://doi.org/10.1038/s42255-019-0089-9
Liu J, Luo G, Sun J et al (2019) METTL14 is essential for β-cell survival and insulin secretion. Biochim Biophys Acta Mol Basis Dis 1865:2138–2148. https://doi.org/10.1016/j.bbadis.2019.04.011
Ping XL, Sun BF, Wang L et al (2014) Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res 24:177–189. https://doi.org/10.1038/cr.2014.3
Frye M, Harada BT, Behm M, He C (2018) RNA modifications modulate gene expression during development. Science 361:1346–1349. https://doi.org/10.1126/science.aau1646
Yue Y, Liu J, He C (2015) RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev 29:1343–1355. https://doi.org/10.1101/gad.262766.115
Wang Y, Gao M, Zhu F et al (2020) METTL3 is essential for postnatal development of brown adipose tissue and energy expenditure in mice. Nat Commun 11:1648. https://doi.org/10.1038/s41467-020-15488-2
Li X, Yuan B, Lu M et al (2021) The methyltransferase METTL3 negatively regulates nonalcoholic steatohepatitis (NASH) progression. Nat Commun 12:7213. https://doi.org/10.1038/s41467-021-27539-3
Li X, Ding K, Li X et al (2022) Deficiency of WTAP in hepatocytes induces lipoatrophy and non-alcoholic steatohepatitis (NASH). Nat Commun 13:4549. https://doi.org/10.1038/s41467-022-32163-w
Wang Y, Sun J, Lin Z et al (2020) m6A mRNA methylation controls functional maturation in neonatal murine β-cells. Diabetes 69:1708–1722. https://doi.org/10.2337/db19-0906
Li X, Yang Y, Chen Z (2023) Downregulation of the m6A reader protein YTHDC1 leads to islet β-cell failure and diabetes. Metab Clin Exp 138:155339. https://doi.org/10.1016/j.metabol.2022.155339
Wang Y, Li X, Liu C et al (2022) WTAP regulates postnatal development of brown adipose tissue by stabilizing METTL3 in mice. Life Metab. https://doi.org/10.1093/lifemeta/loac028
Herrera PL (2000) Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development 127:2317–2322. https://doi.org/10.1242/dev.127.11.2317
Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB (2000) Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49:424–430. https://doi.org/10.2337/diabetes.49.3.424
Chen Z, Morris DL, Jiang L, Liu Y, Rui L (2014) SH2B1 in β-cells regulates glucose metabolism by promoting β-cell survival and islet expansion. Diabetes 63:585–595. https://doi.org/10.2337/db13-0666
Ngara M, Wierup N (2022) Lessons from single-cell RNA sequencing of human islets. Diabetologia 65:1241–1250. https://doi.org/10.1007/s00125-022-05699-1
Donath MY, Dalmas E, Sauter NS, Boni-Schnetzler M (2013) Inflammation in obesity and diabetes: islet dysfunction and therapeutic opportunity. Cell Metab 17:860–872. https://doi.org/10.1016/j.cmet.2013.05.001
Liu J, Yue Y, Han D et al (2014) A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol 10:93–95. https://doi.org/10.1038/nchembio.1432
Dominissini D, Moshitch-Moshkovitz S, Schwartz S et al (2012) Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485:201–206. https://doi.org/10.1038/nature11112
Li X, Wu Y, Song Y et al (2020) Activation of NF-κB-Inducing kinase in islet β cells causes β cell failure and diabetes. Mol Ther 28:2430–2441. https://doi.org/10.1016/j.ymthe.2020.07.016
Johnson JD, Ahmed NT, Luciani DS et al (2003) Increased islet apoptosis in Pdx1+/- mice. J Clin Investig 111:1147–1160. https://doi.org/10.1172/JCI200316537
Acknowledgements
We thank M. Liu (Tianjin Medical University General Hospital, China) for providing RIP-Cre mice. We also thank Y. Han (Novogene Co., China) for assistance in RNA-seq and MeRIP-seq experiments.
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
XL and YY performed the experiments, collected data, analysed and interpreted data, and drafted the manuscript. ZL, YW and JQ analysed and interpreted data, and revised the manuscript. ZC designed the project and wrote the manuscript. ZC is the guarantor of this work. All authors approved the final version to be published.
Funding
This study was supported by the National Natural Science Foundation of China Grant (92057110 and 31971083 to ZC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Yang, Y., Li, Z. et al. Deficiency of WTAP in islet beta cells results in beta cell failure and diabetes in mice. Diabetologia 66, 1084–1096 (2023). https://doi.org/10.1007/s00125-023-05900-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-023-05900-z