Abstract
Summary
Kefir treatment in ovariectomized (OVX) rats could significantly decrease the levels of bone turnover markers and prevent OVX-induced bone loss, deterioration of trabecular microarchitecture, and biomechanical dysfunction that may be due to increase intracellular calcium uptake through the TRPV6 calcium channel.
Introduction
Osteoporosis is a disease characterized by low bone mass and structural deterioration of bone tissue, leading to an increased fracture risk. The incidence of osteoporosis increases with age and occurs most frequently in postmenopausal women due to estrogen deficiency, as the balance between bone resorption and bone formation shifts towards increased levels of bone resorption. Among various methods of prevention and treatment for osteoporosis, an increase in calcium intake is the most commonly recommended preventive measure. Kefir is a fermented milk product made with kefir grains that degrade milk proteins into various peptides with health-promoting effects, including immunomodulating-, antithrombotic-, antimicrobial-, and calcium-absorption-enhancing bioactivities.
Methods
The aim of this study is to investigate the effect of kefir on osteoporosis prophylaxis in an ovariectomized rat model. A total of 56 16-week-old female Sprague-Dawley (SD) rats were divided into 7 experimental groups: sham (normal), OVX/Mock, OVX/1X kefir (164 mg/kg BW/day), OVX/2X kefir (328 mg/kg BW/day), OVX/4X kefir (656 mg/kg BW/day), OVX/ALN (2.5 mg/kg BW/day), and OVX/REBONE (800 mg/kg BW/day). After 12-week treatment with kefir, the bone physiology in the OVX rat model was investigated. Accordingly, the aim of this study was to investigate the possible transport mechanism involved in calcium absorption using the Caco-2 human cell line.
Results
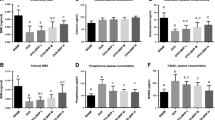
A 12-week treatment with kefir on the OVX-induced osteoporosis model reduced the levels of C-terminal telopeptides of type I collagen (CTx), bone turnover markers, and trabecular separation (Tb. Sp.). Additionally, treatment with kefir increased trabecular bone mineral density (BMD), bone volume (BV/TV), trabecular thickness (Tb. Th), trabecular number (Tb. N), and the biomechanical properties (hardness and modulus) of the distal femur with a dose-dependent efficacy. In addition, in in vitro assay, we found that kefir increased intracellular calcium uptake in Caco-2 cell through TRPV6 calcium channels and not through L-type voltage-operated calcium channels.
Conclusion
The protective effect of kefir in the OVX rat model may occur through increasing intracellular calcium uptake through the TRPV6 calcium channel.






Similar content being viewed by others
References
Renno AC, de Moura FM, Dos SN, Tirico RP, Bossini PS, Parizotto NA (2006) Effects of 830-nm laser, used in two doses, on biomechanical properties of osteopenic rat femora. Photomed Laser Surg 24:202–206
Genant HK, Delmas PD, Chen P, Jiang Y, Eriksen EF, Dalsky GP, Marcus R, San MJ (2007) Severity of vertebral fracture reflects deterioration of bone microarchitecture. Osteoporos Int 18:69–76
Chen H, Wu M, Kubo KY (2012) Combined treatment with a traditional Chinese medicine, Hachimi-jio-gan (Ba-Wei-Di-Huang-Wan) and alendronate improves bone microstructure in ovariectomized rats. J Ethnopharmacol 142:80–85
Naot D, Grey A, Reid IR, Cornish J (2005) Lactoferrin—a novel bone growth factor. Clin Med Res 5:93–101
Shen Y, Li YQ, Li SP, Ma L, Ding LJ, Ji H (2010) Alleviation of ovariectomy-induced osteoporosis in rats by Panax notoginseng saponins. J Nat Med 64(3):336–345
Turner CH, Burr DB (1993) Basic biochemical measurements of bone: a tutorial. Bone 14:595–608
Cho DC, Kim KT, Jeon Y, Sung JK (2012) A synergistic bone sparing effect of curcumin and alendronate in ovariectomized rat. Acta Neurochir 154(12):2215–2223
Zhao X, Wu ZX, Zhang Y, Yan YB, He Q, Cao PC, Lei W (2011) Anti-osteoporosis activity of Cibotium barometz extract on ovariectomy-induced bone loss in rats. J Ethnopharmacol 137(3):1083–1088
Lopitz-Otsoa F, Rementeria A, Elguezabal N, Garaizar J (2006) Kefir: a symbiotic yeasts-bacteria community with alleged healthy capabilities. Rev Iberoam Micol 23:67–74
Guzel-Seydim ZB, Kok-Tas T, Greene AK, Seydim AC (2011) Review: functional properties of kefir. Crit Rev Food Sci Nutr 51:261–268
de de Moreno AL, Matar C, Farnworth E, Perdigon G (2007) Study of immune cells involved in the antitumor effect of kefir in a murine breast cancer model. J Dairy Sci 90:1920–1928
Chen HL, Tung YT, Tsai CL, Lai CW, Tsai HC, Lin YL, Wang CH, Chen CM (2014) Kefir improves fatty liver syndrome through inhibited lipogenesis pathway in leptin-deficient ob/ob knockout mice. Int J Obesity 38:1172–1179
Vinderola G, Perdigón G, Duarte J, Farnworth E, Matar C (2006) Effects of the oral administration of the products derived from milk fermentation by kefir microflora on immune stimulation. J Dairy Res 73:472–479
Wronski TJ, Lowry PL, Walsh CC, Ignaszewski LA (1985) Skeletal alterations in ovariectomized rats. Calcif Tissue Int 37:324–328
Wonski TJ, Dann LM, Scott KS, Crooke LR (1989) Endocrine and pharmacological suppressors of bone turnover protect against osteopenia in ovariectomized rats. Endocrinology 25:810–816
Kalu DK (1991) The ovariectomized rat model of postmenopausal bone loss. J Bone Miner 15:175–192
Rocha RA, Devesa V, Vélez D (2013) In vitro study of intestinal transport of fluoride using the Caco-2 cell line. Food Chem Toxicol 55:156–163
Hidalgo IJ, Raub TJ, Borchardt RT (1989) Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96:736–749
Maubon N, Le Vee M, Fossati L, Audry M, Le Ferrec E, Bolze S, Fardel O (2007) Analysis of drug transporter expression in human intestinal Caco-2 cells by real-time PCR. Fundam Clin Pharmacol 21:659–663
Lin CW, Chen HL, Liu JR (1999) Identification and characterization of lactic acid bacteria and yeasts isolated from kefir grains in Taiwan. Aust J Dairy Technol 54:14–18
Ko YJ, Wu JB, Ho HY, Lin WC (2012) Antiosteoporotic activity of Davallia formosana. J Ethnopharmacol 139(2):558–565
Shackelford C, Long G, Wolf J, Okerberg C, Herbert R (2002) Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol Pathol 30(1):93–96
Wang YT, Chang SY, Huang YC, Tsai TC, Chen CM, Lim CT (2013) Nanomechanics insights into the performance of healthy and osteoporotic bones. Nano Lett 13(11):5247–5254
Chang YT, Chen CM, Tu MY, Chen HL, Chang SY, Tsai TC, Wang YT, Hsiao HL (2011) Effects of osteoporosis and nutrition supplements on structures and nanomechanical properties of bone tissue. J Mech Behav Biomed Mater 4:1412–1420
McCormack JG, Cobbold PH (1991) Cellular calcium: a practical approach. Oxford University Press, New York, NY, The practical approach series
Hertrampf T, Schleipen B, Velders M, Laudenbach U, Fritzemeier KH, Diel P (2008) Estrogen receptor subtype-specific effects on markers of bone homeostasis. Mol Cell Endocrinol 291:104–108
Jagtap VR, Ganu JV, Nagane NS (2011) BMD and serum intact osteocalcin in postmenopausal osteoporosis women. Indian J Clin Biochem 26:70–73
Kim T, Ha H, Shim KS, Cho WK, Ma JY (2013) The anti-osteoporotic effect of Yijung-tang in an ovariectomized rat model mediated by inhibition of osteoclast differentiation. J Ethnopharmacol 146(1):83–89
Calvo MS, Eyre DR, Gundberg CM (1996) Molecular basis and clinical application of biological markers of bone turnover. Endocr Rev 17:33–368
Garnero P, Delmas PD (1998) Biochemical makers of bone turnover: applications for osteoporosis. Endocrinol Metab Clin N Am 27:303–323
Rosen HN, Moses AC, Garber J, Iloputaife ID, Ross DS, Lee SL, Greenspan SL (2000) Serum CTX: a new marker of bone resorption that shows treatment effect more than other markers because of low coefficient of variability and large changes with bisphosphonate therapy. Calcif Tissue Int 66:100–103
Garnero P (1996) Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res 11:337–349
Melton L (1997) Relationship of bone turnover to bone density and fractures. J Bone Miner Res 12:1083–1091
Bouxsein ML (2003) Mechanisms of osteoporosis therapy: a bone strength perspective. Clin Cornerstone S13–S21
Peng ZQ, Vaananen HK, Zhang HX, Tuukkanen J (1997) Long-term effects of ovariectomy on the mechanical properties and chemical composition of rat bone. Bone 20:207–212
Urasopon N, Hamada Y, Cherdshewasart W, Malaivijitnond S (2008) Preventive effects of Pueraria mirifica on bone loss in ovariectomized rats. Maturitas 59:137–148
Zhang R, Liu ZG, Li C, Hu SJ, Liu L, Wang JP, Mei QB (2009) Du-Zhong (Eucommia ulmoides Oliv.) cortex extract prevent OVX-induced osteoporosis in rats. Bone 45:553–559
Snyde BD, Piazza S, Edwards WT, Hayes WC, Schaffler R (1993) Role of trabecular morphology in the etiology of age-related vertebral fractures. Calcif Tissue International 53:14–22
Kleerekope M, Villanueva AR, Stanciu J, Rao DS, Parfitt AM (1995) The role of three-dimensional trabecular microstructure in the pathogenesis of vertebral compression fractures. Calcif Tissue Int 37:594–597
Laib A, Barou O, Vico L, Lafage-Proust MH, Alexandre C, Rugsegger P (2000) 3D micro-computed tomography of trabecular and cortical bone architecture with application to a rat model of immobilisation osteoporosis. Med Biol Eng Comput 38:326–332
Sran MM, Boyd SK, Cooper DM, Khan KM, Zernicke RF, Oxland TR (2007) Regional trabecular morphology assessed by micro-CT is correlated with failure of aged thoracic vertebrae under a posteroanterior load and may determine the site of fracture. Bone 40:751–757
Yarrow JF, Conover CF, Purandare AV, Bhakta AM, Zheng N, Conrad B, Altman MK, Franz SE, Wronski TJ, Borst SE (2008) Supraphysiological testosterone enanthate administration prevents bone loss and augments bone strength in gonadectomized male and female rats. Am J Physiol Endocrinol Metab 295:E1213–E1222
Libouban H, Blouin S, Moreau MF, Baslé MF, Audran M, Chappard D (2008) Effects of risedronate in a rat model of osteopenia due to orchidectomy and disuse: densitometric, histomorphometric and microtomographic studies. Micron 39:998–1007
Kruger MC, Chua WH, Darragh A, Booth CL, Prosser C, Lowry D (2008) Impact of goat milk powdered formulations on mineral absorption, peak bone mass and bone loss due to ovariectomy in rats. J Sci Food Agric 88:1082–1090
Bonjour JP, Benoit V, Payen F, Kraenzlin M (2013) Consumption of yogurts fortified in vitamin D and calcium reduces serum parathyroid hormone and markers of bone resorption: a double-blind randomized controlled trial in institutionalized elderly women. J Clin Endocrinol Metab 98:2015–2021
Narva M, Rissanen J, Halleen J, Vapaatalo H, Väänänen K, Korpela R (2007) Effects of bioactive peptide, valyl-prolyl-proline (VPP), and lactobacillus helveticus fermented milk containing VPP on bone loss in ovariectomized rats. Ann Nutr Metab 51(1):65–74
Acknowledgments
This research was supported in part by grants NSC101-2324-B-005-002-CC1 from the National Science Council and the Ministry of Education, Taiwan, Republic of China, under the Aiming for Top University plan (ATU-101-s0508). The authors would like to thank our colleague, Professor Jiunn-Wang Liao in the Graduate Institute of Veterinary Pathology, National Chung Hsing university, for his help with the discussions and technical issues.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
H.-L. Chen, Y.-T. Tung, C.-H. Chuang, and M.-Y. Tu contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
The reproducibility of kefir. They were separated by semipreparative HPLC on a model PU-980 pump (Jasco, Japan) equipped with a UV detector and a 300 × 7.8 mm i.d., 5-μm particles TSK-GEL G2000SWXL column (Sigma-Aldrich, St Louis, MO). The mobile phase was 100 mM KH2PO4, 1 M NaCl and 1 mM EDTA (pH = 6.5) at a flow rate of 0.5 mL/min, and the wavelength was detected at 215 nm (TIFF 7478 kb) (GIF 48 kb)
Rights and permissions
About this article
Cite this article
Chen, HL., Tung, YT., Chuang, CH. et al. Kefir improves bone mass and microarchitecture in an ovariectomized rat model of postmenopausal osteoporosis. Osteoporos Int 26, 589–599 (2015). https://doi.org/10.1007/s00198-014-2908-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-014-2908-x