Abstract
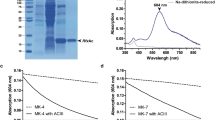
The green sulfur bacterium, Chlorobaculum tepidum, is an anaerobic photoautotroph that performs anoxygenic photosynthesis. Although genes encoding rubredoxin (Rd) and a putative flavodiiron protein (FDP) were reported in the genome, a gene encoding putative NADH-Rd oxidoreductase is not identified. In this work, we expressed and purified the recombinant Rd and FDP and confirmed dioxygen reductase activity in the presence of ferredoxin-NAD(P)+ oxidoreductase (FNR). FNR from C. tepidum and Bacillus subtilis catalyzed the reduction of Rd at rates comparable to those reported for NADH-Rd oxidoreductases. Also, we observed substrate inhibition at high concentrations of NADPH similar to that observed with ferredoxins. In the presence of NADPH, B. subtilis FNR and Rd, FDP promoted dioxygen reduction at rates comparable to those reported for other bacterial FDPs. Taken together, our results suggest that Rd and FDP participate in the reduction of dioxygen in C. tepidum and that FNR can promote the reduction of Rd in this bacterium.



Similar content being viewed by others
References
Alboresi A, Storti M, Cendron L, Morosinotto T (2019) Role and regulation of class-C flavodiiron proteins in photosynthetic organisms. Biochem J 476:2487–2498
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Anbar AD, Duan Y, Lyons TW, Arnold GL, Kendall B, Creaser RA, Kaufman AJ, Gordon GW, Scott C, Garvin J, Buick R (2007) A whiff of oxygen before the great oxidation event? Science 317:1903–1906
Argyrou A, Blanchard JS (2004) Flavoprotein disulfide reductases: advances in chemistry and function. Prog Nucleic Acid Res Mol Biol 78:89–142
Bertsova YV, Kulik LV, Mamedov MD, Baykov AA, Bogachev AV (2019a) Flavodoxin with an air-stable flavin semiquinone in a green sulfur bacterium. Photosynth Res 142:127–136
Bertsova YV, Mamedov MD, Bogachev AV (2019b) Na+-Translocating Ferredoxin:NAD+ oxidoreductase is a component of photosynthetic electron transport chain in green sulfur bacteria. Biochemistry (Mosc) 84:1403–1410
Brummett AE, Dey M (2016) New Mechanistic insight from substrate- and product-bound structures of the metal-dependent dimethylsulfoniopropionate lyase DddQ. Biochemistry 55:6162–6174
Buchanan BB, Arnon DI (1971) Ferredoxins from photosynthetic bacteria, algae, and higher plants. Methods Enzymol 23(C):413–440
Buchanan BB, Arnon DI (1990) A reverse KREBS cycle in photosynthesis: consensus at last. Photosyn Res 24:47–53
Buchanan BB, Sirevåg R, Fuchs G, Ivanovsky RN, Igarashi Y, Ishii M, Tabita FR, Berg IA (2017) The Arnon-Buchanan cycle: a retrospective, 1966–2016. Photosynth Res 134117–134131
Cabeza MS, Guerrero SA, Iglesias AA, Arias DG (2015) New enzymatic pathways for the reduction of reactive oxygen species in Entamoeba histolytica. Biochim Biophys Acta 1850:1233–1244
Chen L, Liu MY, Legall J, Fareleira P, Santos H, Xavier AV (1993) Purification and characterization of an NADH-rubredoxin oxidoreductase involved in the utilization of oxygen by Desulfovibrio gigas. Eur J Biochem 216:443–448
Dey M, Brummett AE (2018) Isolation and assays of bacterial dimethylsulfoniopropionate lyases. Methods Enzymol 605:291–323
Di Matteo A, Scandurra FM, Testa F, Forte E, Sarti P, Brunori M, Giuffrè A (2008) The O2-scavenging flavodiiron protein in the human parasite Giardia intestinalis. J Biol Chem 283:4061–4068
Dym O, Eisenberg D (2001) Sequence-structure analysis of FAD-containing proteins. Protein Sci 10:1712–1728
Eddie BJ, Hanson TE (2013) Chlorobaculum tepidum TLS displays a complex transcriptional response to sulfide addition. J Bacteriol 195:399–408
Eisen JA, Nelson KE, Paulsen IT, Heidelberg JF, Wu M, Dodson RJ, Deboy R, Gwinn ML, Nelson WC, Haft DH, Hickey EK, Peterson JD, Durkin AS, Kolonay JL, Yang F, Holt I, Umayam LA, Mason T, Brenner M, Shea TP, Parksey D, Nierman WC, Feldblyum TV, Hansen CL, Craven MB, Radune D, Vamathevan J, Khouri H, White O, Gruber TM, Ketchum KA, Venter JC, Tettelin H, Bryant DA, Fraser CM (2002) The complete genome sequence of Chlorobium tepidum TLS, a photosynthetic, anaerobic, green-sulfur bacterium. Proc Natl Acad Sci U S A 99:9509–9514
Evans MC, Buchanan BB, Arnon DI (1966) A new ferredoxin-dependent carbon reduction cycle in a photosynthetic bacterium. Proc Natl Acad Sci U S A 55:928–934
Fang H, Caranto JD, Mendoza R, Taylor AB, Hart PJ, Kurtz DM Jr (2012) Histidine ligand variants of a flavo-diiron protein: effects on structure and activities. J Biol Inorg Chem 17:1231–1239
Folgosa F, Martins MC, Teixeira M (2018) Diversity and complexity of flavodiiron NO/O2 reductases. FEMS Microbiol Lett. 365–267
Frazão C, Silva G, Gomes CM, Matias P, Coelho R, Sieker L, Macedo S, Liu MY, Oliveira S, Teixeira M, Xavier AV, Rodrigues-Pousada C, Carrondo MA, Le Gall J (2000) Structure of a dioxygen reduction enzyme from Desulfovibrio gigas. Nat Struct Biol 11:1041–1045
Frederick RE, Caranto JD, Masitas CA, Gebhardt LL, MacGowan CE, Limberger RJ, Kurtz DM Jr (2015) Dioxygen and nitric oxide scavenging by Treponema denticola flavodiiron protein: a mechanistic paradigm for catalysis. J Biol Inorg Chem 20:603–613
Fukuyama K (2004) Structure and function of plant-type ferredoxins. Photosyn Res 81:289–301
Geueke B, Riebel B, Hummel W (2003) NADH oxidase from Lactobacillus brevis: a new catalyst for the regeneration of NAD. Enz Microbial Tech 32:205–211
Gregersen LH, Bryant DA, Frigaard NU (2011) Mechanisms and evolution of oxidative sulfur metabolism in green sulfur bacteria. Front Microbiol 24:116
Hagelueken G, Wiehlmann L, Adams TM, Kolmar H, Heinz DW, Tümmler B, Schubert WD (2007) Crystal structure of the electron transfer complex rubredoxin rubredoxin reductase of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 104:12276–12281
Hillmann F, Riebe O, Fischer RJ, Mot A, Caranto JD, Kurtz DM Jr, Bahl H (2009) Reductive dioxygen scavenging by flavo-diiron proteins of Clostridium acetobutylicum. FEBS Lett 583:241–245
Jokel M, Kosourov S, Battchikova N, Tsygankov AA, Aro EM, Allahverdiyeva Y (2015) Chlamydomonas Flavodiiron proteins facilitate acclimation to anoxia during sulfur deprivation. Plant Cell Physiol 56:1598–1607
Kawasaki S, Ishikura J, Chiba D, Nishino T, Niimura Y (2004) Purification and characterization of an H2O-forming NADH oxidase from Clostridium aminovalericum: existence of an oxygen-detoxifying enzyme in an obligate anaerobic bacteria. Arch Microbiol 181:324–330
Kawasaki S, Sakai Y, Takahashi T, Suzuki I, Niimura Y (2009) O2 and reactive oxygen species detoxification complex, composed of O2-responsive NADH: rubredoxin oxidoreductase-flavoprotein A2-desulfoferrodoxin operon enzymes, rubperoxin, and rubredoxin, in Clostridium acetobutylicum. Appl Environ Microbiol 75:1021–1029
Kjær B, Scheller HV (1996) An isolated reaction center complex from the green sulfur bacterium Chlorobium vibrioforme can photoreduce ferredoxin at high rates. Photosyn Res 47:33–39
Knaff DB, Hirasawa M (1991) Ferredoxin-dependent chloroplast enzymes. Biochim Biophys Acta 1056:93–125
Kusumoto N, Inoue K, Nasu H, Sakurai H (1994) Preparation of a photoactive reaction center complex containing photo-reducible Fe-S centers and photooxidizable cytochrome c from the green sulfur bacterium Chlorobium tepidum. Plant Cell Phys 35:17–25
Lee HJ, Basran J, Scrutton NS (1998) Electron transfer from flavin to iron in the Pseudomonas oleovorans rubredoxin reductase-rubredoxin electron transfer complex. Biochemistry 37:15513–15522
Li H, Jubelirer S, Garcia Costas AM, Frigaard NU, Bryant DA (2009) Multiple antioxidant proteins protect Chlorobaculum tepidum against oxygen and reactive oxygen species. Arch Microbiol 191:853–867
Lountos GT, Jiang R, Wellborn WB, Thaler TL, Bommarius AS, Orville AM (2006) The crystal structure of NAD(P)H oxidase from Lactobacillus sanfranciscensis: insights into the conversion of O2 into two water molecules by the flavoenzyme. Biochemistry 45:9648–9659
Ma K, Adams MW (1999) A hyperactive NAD(P)H: rubredoxin oxidoreductase from the hyperthermophilic archaeon Pyrococcus furiosus. J Bacteriol 181:5530–5533
Maiti BK, Almeida RM, Moura I, Moura JJG (2017) Rubredoxins derivatives: simple sulphur-rich coordination metal sites and its relevance for biology and chemistry. Coordination Chem Rev 352:379–397
Martins MC, Romão CV, Folgosa F, Borges PT, Frazão C, Teixeira M (2019) How superoxide reductases and flavodiiron proteins combat oxidative stress in anaerobes. Free Radic Biol Med 140:36–60
Medina M, Gómez-Moreno C (2004) Interaction of ferredoxin-NADP+ reductase with its substrates: optimal interaction for efficient electron transfer. Photosynth Res 79:113–131
Miller M, Liu X, Snyder SW, Thurnauer MC, Biggins J (1992) Photosynthetic electron-transfer reactions in the green sulfur bacterium Chlorobium vibrioforme: evidence for the functional involvement of iron-sulfur redox centers on the acceptor side of the reaction center. Biochemistry 31:4354–4363
Notredame C, Higgins DH, Heringa J (2000) T-Coffee: a novel method for multiple sequence alignments. J Mol Biol 302:205–217
Ohnishi K, Niimura Y, Yokoyama K, Hidaka M, Masaki H, Uchimura T, Suzuki H, Uozumi T, Kozaki M, Komagata K, Nishino T (1994) Purification and analysis of a flavoprotein functional as NADH oxidase from Amphibacillus xylanus overexpressed in Escherichia coli. J Biol Chem 269:31418–31423
Okegawa Y, Motohashi K (2015) A simple and ultra-low cost homemade seamless ligation cloning extract (SLiCE) as an alternative to a commercially available seamless DNA cloning kit. Biochem Biophys Rep 4:148–151
Romão CV, Vicente JB, Borges PT, Frazão C, Teixeira M (2016a) The dual function of flavodiiron proteins: oxygen and/or nitric oxide reductases. J Biol Inorg Chem 21:39–52
Romão CV, Vicente JB, Borges PT, Victor BL, Lamosa P, Silva E, Pereira L, Bandeiras TM, Soares CM, Carrondo MA, Turner D, Teixeira M, Frazão C (2016b) Structure of Escherichia coli flavodiiron nitric oxide reductase. J Mol Biol 428:4686–4707
Sakurai H, Ogawa T, Shiga M, Inoue K (2010) Inorganic sulfur oxidizing system in green sulfur bacteria. Photosynth Res 104:163–176
Saunders AH, Golbeck JH, Bryant DA (2013) Characterization of BciB: a ferredoxin-dependent 8-vinyl-protochlorophyllide reductase from the green sulfur bacterium Chloroherpeton thalassium. Biochemistry 52:8442–8451
Seo D, Sakurai H (2002) Purification and characterization of ferredoxin-NAD(P)+ reductase from the green sulfur bacterium Chlorobium tepidum. Biochim Biophys Acta 1597:123–132
Seo D, Tomioka A, Kusumoto N, Kamo M, Enami I, Sakurai H (2001) Purification of ferredoxins and their reaction with purified reaction center complex from the green sulfur bacterium Chlorobium tepidum. Biochim Biophy Acta 1503:377–384
Seo D, Kamino K, Inoue K, Sakurai H (2004) Purification and characterization of ferredoxin-NADP+ reductase encoded by Bacillus subtilis yumC. Arch Microbiol 182:80–89
Seo D, Okabe S, Yanase M, Kataoka K, Sakurai T (2009) Studies of interaction of homo-dimeric ferredoxin-NAD(P)+ oxidoreductases of Bacillus subtilis and Rhodopseudomonas palustris, that are closely related to thioredoxin reductases in amino acid sequence, with ferredoxins and pyridine nucleotide coenzymes. Biochim Biophys Acta 1794:594–601
Seo D, Asano T, Komori H, Sakurai T (2014) Role of the C-terminal extension stacked on the re-face of the isoalloxazine ring moiety of the flavin adenine dinucleotide prosthetic group in ferredoxin-NADP+ oxidoreductase from Bacillus subtilis. Plant Physiol Biochem 81:143–148
Seo D, Soeta T, Sakurai H, Sétif P, Sakurai T (2016a) Pre-steady-state kinetic studies of redox reactions catalyzed by Bacillus subtilis ferredoxin-NADP+ oxidoreductase with NADP+/NADPH and ferredoxin. Biochim Biophys Acta 1857:678–687
Seo D, Kitashima M, Sakurai T, Inoue K (2016b) Kinetics of NADP+/NADPH reduction-oxidation catalyzed by the ferredoxin-NAD(P)+ reductase from the green sulfur bacterium Chlorobaculum tepidum. Photosynth Res 130:479–489
Sétif P (2001) Ferredoxin and flavodoxin reduction by photosystem I. Biochim Biophys Acta 1507:161–179
Tanaka M, Haniu M, Evans MCW, Rao KK (1974) Amino acid sequence of ferredoxin from a photosynthetic green bacterium, Chlorobium limicola. Biochemistry 13:2953–2959
Tanaka M, Haniu M, Yasunobu KT, Evans MCW, Rao KK (1975) The amino acid sequence of ferredoxin II from Chlorobium limicola, a photosynthetic green bacterium. Biochemistry 14:1938–1943
Tang KH, Blankenship RE (2010) Both forward and reverse TCA cycles operate in green sulfur bacteria. J Biol Chem 285:35848–35854
Tsukatani Y, Miyamoto R, Itoh S, Oh-oka H (2004) Function of a PscD subunit in a homodimeric reaction center complex of the photosynthetic green sulfur bacterium Chlorobium tepidum studied by insertional gene inactivation: regulation of energy transfer and ferredoxin-mediated NADP+ reduction on the cytoplasmic side. J Biol Chem 279:51122–51130
Vassiliev IR, Ronan MT, Hauska G, Golbeck JH (2000) The bound electron acceptors in green sulfur bacteria: resolution of the g-tensor for the F(X) iron-sulfur cluster in Chlorobium tepidum. Biophys J 78:3160–3169
Vicente JB, Carrondo MA, Teixeira M, Frazão C (2008) Structural studies on flavodiiron proteins. Methods Enzymol 437:3–19
Vicente JB, Tran V, Pinto L, Teixeira M, Singh U (2012) A detoxifying oxygen reductase in the anaerobic protozoan Entamoeba histolytica. Eukaryot Cell 11:1112–1118
Wahlund TM, Madigan MT (1993) Nitrogen fixation by the thermophilic green sulfur bacterium Chlorobium tepidum. J Bacteriol 175:474–478
Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L, Lepore R, Schwede T (2018) SWISS-MODEL: homology modeling of protein structures and complexes. Nucleic Acids Res 46:W296–W303
Yoon KS, Hille R, Hemann C, Tabita FR (1999) Rubredoxin from the green sulfur bacterium Chlorobium tepidum functions as an electron acceptor for pyruvate ferredoxin oxidoreductase. J Biol Chem 274:29772–29778
Yoon KS, Bobst C, Hemann CF, Hille R, Tabita FR (2001) Spectroscopic and functional properties of novel 2[4Fe-4S] cluster-containing ferredoxins from the green sulfur bacterium Chlorobium tepidum. J Biol Chem 276:44027–44036
Acknowledgements
The authors would like to thank ENAGO for the English language review.
Funding
This work was partly supported by Japan Society for the Promotion of Science KAKENHI Grant Number JP17K07304 (to DS) and JP18K06296 (to KI).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Erko stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ittarat, W., Sato, T., Kitashima, M. et al. Rubredoxin from the green sulfur bacterium Chlorobaculum tepidum donates a redox equivalent to the flavodiiron protein in an NAD(P)H dependent manner via ferredoxin-NAD(P)+ oxidoreductase. Arch Microbiol 203, 799–808 (2021). https://doi.org/10.1007/s00203-020-02079-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-020-02079-4