Abstract
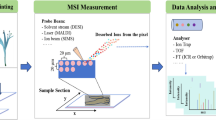
Advanced analytical imaging techniques, including matrix-assisted laser desorption/ionization high-resolution mass spectrometry (MALDI-HRMS) imaging, can be used to visualize the distribution, localization, and dynamics of target compounds and their precursors with limited sample preparation. Herein we report an application of MALDI-HRMS imaging to map, in high spatial resolution, the accumulation of the medicinally important naphthodianthrone hypericin, its structural analogues and proposed precursors, and other crucial phytochemical constituents in the leaves of two hypericin-containing species, Hypericum perforatum and Hypericum olympicum. We also investigated Hypericum patulum, which does not contain hypericin or its protoforms. We focused on both the secretory (dark glands, translucent glands, secretory canals, laminar glands, and ventral glands) and the surrounding non-secretory tissues to clarify the site of biosynthesis and localization of hypericin, its possible precursors, and patterns of localization of other related compounds concomitant to the presence or absence of hypericin. Hypericin, pseudohypericin, and protohypericin accumulate in the dark glands. However, the precursor emodin not only accumulates in the dark glands but is also present outside the glands in both hypericin-containing species. In hypericin-lacking H. patulum, however, emodin typically accumulates only in the glands, thereby providing evidence that hypericin is possibly biosynthesized outside the dark glands and thereafter stored in them. The distribution and localization of related compounds were also evaluated and are discussed concomitant to the occurrence of hypericin. Our study provides the basis for further detailed investigation of hypericin biosynthesis by gene discovery and expression studies.






Similar content being viewed by others
References
Kosuth J, Smelcerovic A, Borsch T, Zuehlke S, Karppinen K, Spiteller M, Hohtola A, Cellarova E (2011) The hyp-1 gene is not a limiting factor for hypericin biosynthesis in the genus Hypericum. Funct Plant Biol 38:35–43
Kosuth J, Hrehorova D, Jaskolski M, Cellarova E (2013) Stress-induced expression and structure of the putative gene hyp-1 for hypericin biosynthesis. Plant Cell Tissue Organ Cult 114:207–216
Kusari S, Lamshöft M, Zühlke S, Spiteller M (2008) An endophytic fungus from Hypericum perforatum that produces hypericin. J Nat Prod 71:159–162
Kusari S, Zühlke S, Kosuth J, Cellarova E, Spiteller M (2009) Light-independent metabolomics of endophytic Thielavia subthermophila provides insight into microbial hypericin biosynthesis. J Nat Prod 72:1825–1835
Crockett SL, Robson NKB (2011) Taxonomy and chemotaxonomy of the genus Hypericum. Med Aromat Plant Sci Biotechnol 5:1–13
Brockmann H, Haschad MN, Maier K, Pohl F (1939) Über das Hypericin, den photodynamisch wirksamen Farbstoff aus Hypericum perforatum. Naturwissenschaften 27:550
Brockmann H, Pohl F, Maier K, Haschad MN (1942) Über das Hypericin, den photodynamischen Farbstoff des Johanniskrautes (Hypericum perforatum). Ann Chem 553:1–52
Brockmann H, Kluge F, Muxfeldt H (1957) Totalsynthese des Hypericins. Chem Ber 90:2302–2318
Nahrstedt A, Butterweck V (1997) Biologically active and other chemical constituents of the herb of Hypericum perforatum L. Pharmacopsychiatry 30:129–134
Häberlein H, Tschiersch KP, Stock S, Hölzl J (1992) Johanniskraut (Hypericum perforatum L.): Nachweis eines weiteren Naphthodianthrons. Pharm Ztg Wiss 5(137):169–174
Kitanov GM (2001) Hypericin and pseudohypericin in some Hypericum species. Biochem Syst Ecol 29:171–178
Makovetska OY (1999) Research of biologically active substances of Hypericum L. species: report II. Farm Zh (Kiev) 1:47–52
Crockett SL, Schaneberg B, Khan IA (2005) Phytochemical profiling of New and Old World Hypericum (St. John's Wort) species. Phytochem Anal 16:479–485
Kirakosyan A, Gibson DM, Kaufman PB (2008) The production of dianthrones and phloroglucinol derivatives in St. John’s Wort. In: Ramawat KG, Merillon JM (eds) Bioactive molecules and medicinal plants. Springer, Berlin, pp 149–164
Brockmann H, Falkenhausen EH, Dorlares A (1950) Die Konstitution des Hypericins. Naturwissenschaften 37:540
Thomson RH (1957) Naturally occurring quinones. Butterworths Scientific Publications, London
Bais HP, Vepachedu R, Lawrence CB, Stermitz FR, Vivanco JM (2003) Molecular and biochemical characterization of an enzyme responsible for the formation of hypericin in St. John’s wort (Hypericum perforatum L.). J Biol Chem 278:32413–32422
Kosuth J, Katkovcinova Z, Olexova P, Cellarova E (2007) Expression of the hyp-1 gene in early stages of development of Hypericum perforatum L. Plant Cell Rep 26:211–217
Cellarova E, Daxnerova Z, Kimakova K, Haluskova J (1994) The variability of hypericin content in the regenerants of Hypericum perforatum. Acta Biotechnol 14:267–274
Briskin DP, Leroy A, Gawienowski M (2000) Influence of nitrogen on the production of hypericins by St. John’s wort. Plant Physiol Biochem 38:413–420
Onelli E, Rivetta A, Giorgi A, Bignami M, Cocucci M, Patrignani G (2002) Ultrastructural studies on the developing secretory nodules of Hypericum perforatum. Flora 197:92–102
Hölzl J, Petersen M (2003) Chemical constituents of Hypericum ssp. In: Ernst E (ed) Hypericum: the genus Hypericum (series: medicinal and aromatic plants - industrial profiles), vol 31. Taylor and Francis, London, pp 77–93
Hadjur C, Richard MJ, Parat MO, Jardon P, Favier A (1996) Photodynamic effects of hypericin on lipid peroxidation and antioxidant status in melanoma cells. Photochem Photobiol 64:375–381
Delaey EM, Obermueller R, Zupko I, De Vos D, Falk H, de Witte PA (2001) In vitro study of the photocytotoxicity of some hypericin analogs on different cell lines. Photochem Photobiol 74:164–171
Kamuhabwa AR, Agostinis PM, D’Hallewin MA, Baert L, de Witte PA (2001) Cellular photodestruction induced by hypericin in AY-27 rat bladder carcinoma cells. Photochem Photobiol 74:126–132
Kubin A, Wierrani F, Burner U, Alth G, Grunberger W (2005) Hypericin - the facts about a controversial agent. Curr Pharm Des 11:233–253
Zobayed SMA, Afreen F, Goto E, Kozai T (2006) Plant-environment interactions: accumulation of hypericin in dark glands of Hypericum perforatum. Ann Bot 98:793–804
Maggi F, Ferretti G, Pocceschi N, Meneghini L, Ricciutelli M (2004) Morphological, histochemical and phytochemical investigation of the genus Hypericum of the central Italy. Fitoterapia 75:702–711
Römpp A, Spengler B (2013) Mass spectrometry imaging with high resolution in mass and space. Histochem Cell Biol 139:759–783
Shih C-J, Chen P-Y, Liaw C-C, Lai Y-M, Yang Y-L (2014) Bringing microbial interactions to light using imaging mass spectrometry. Nat Prod Rep. doi:10.1039/c3np70091g
Bjarnholt N, Li B, D’Alvise J, Janfelt C (2014) Mass spectrometry imaging of plant metabolites—principles and possibilities. Nat Prod Rep. doi:10.1039/c3np70100j
Jaeger RJ, Lamshöft M, Gottfried S, Spiteller M, Spiteller P (2013) HR-MALDI-MS imaging assisted screening of β-carboline alkaloids discovered from Mycena metata. J Nat Prod 76:127–134
Kusari P, Kusari S, Lamshöft M, Sezgin S, Spiteller M, Kayser O (2014) Quorum quenching is an antivirulence strategy employed by endophytic bacteria. Appl Microbiol Biotechnol 98:7173–7183
Kusari S, Lamshöft M, Kusari P, Gottfried S, Zühlke S, Louven K, Hentschel U, Kayser O, Spiteller M (2014) Endophytes are hidden producers of maytansine in Putterlickia roots. J Nat Prod 77:2577–2584
Ciccarelli D, Andreucci AC, Pagni AM (2001) Translucent glands and secretory canals in Hypericum perforatum L. (Hypericaceae): morphological, anatomical and histochemical studies during the course of ontogenesis. Ann Bot 88:637–644
Łotocka B, Osińska E (2010) Shoot anatomy and secretory structures in Hypericum species (Hypericaceae). Bot J Linn Soc 163:70–86
Nürk NM, Crockett SL (2011) Morphological and phytochemical diversity among Hypericum species of the Mediterranean Basin. Med Aromat Plant Sci Biotechnol 5:14–28
Perrone R, Rosa PD, Castro OD, Colombo P (2013) Leaf and stem anatomy in eight Hypericum species (Clusiaceae). Acta Bot Croat 72:269–286
Soelberg J, Jørgensen LB, Jäger AK (2007) Hyperforin accumulates in the translucent glands of Hypericum perforatum. Ann Bot 99:1097–1100
Hölscher D, Shroff R, Knop K, Gottschaldt M, Crecelius A, Schneider B, Heckel DG, Schubert US, Svatos A (2009) Matrix-free UV-laser desorption/ionization (LDI) mass spectrometric imaging at the single-cell level: distribution of secondary metabolites of Arabidopsis thaliana and Hypericum species. Plant J 60:907–918
Thunig J, Hansen SH, Janfelt C (2011) Analysis of secondary plant metabolites by indirect desorption electrospray ionization imaging mass spectrometry. Anal Chem 83:3256–3259
Kusari S, Zühlke S, Borsch T, Spiteller M (2009) Positive correlations between hypericin and putative precursors detected in the quantitative secondary metabolite spectrum of Hypericum. Phytochemistry 70:1222–1232
Harborne JB (1993) Introduction to ecological biochemistry, 4th edn. Academic Press, London
Smelcerovic A, Spiteller M (2006) Phytochemical analysis of nine Hypericum L. species from Serbia and the F.Y.R. Macedonia. Pharmazie 61:251–252
Izhaki I (2002) Emodin – a secondary metabolite with multiple ecological functions in higher plants. New Phytol 155:205–217
Cornett DS, Reyzer ML, Chaurand P, Caprioli RM (2007) MALDI imaging mass spectrometry: molecular snapshots of biochemical systems. Nat Methods 4:828–833
Acknowledgments
This research was funded in part by the grant project SOFOS-knowledge and skill development of staff and students of P. J. Safarik University in Kosice (contract number: 003/2013/1.2/OPV, ITMS code: 26110230088), funded by the European Social Fund through the Operational Program Education. S.K. was the tutor assigned to supervise and train K.N. within the scope of the SOFOS grant project. We thank the German Research Foundation (DFG) for financing the MALDI imaging high-resolution mass spectrometers and the Scientific Grant Agency of Slovak Republic VEGA 1/0090/15. We gratefully acknowledge Dr S. Zühlke (INFU, TU Dortmund) for valuable discussions, technical assistance, and critically reviewing our manuscript.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This manuscript is dedicated with best wishes to Professor Dr Christian Hertweck to honor his remarkable recognition as a Gottfried Wilhelm Leibniz Prize winner in 2015.
Published in the topical collection Mass Spectrometry Imaging with guest editors Andreas Römpp and Uwe Karst.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1.35 mb)
Rights and permissions
About this article
Cite this article
Kusari, S., Sezgin, S., Nigutova, K. et al. Spatial chemo-profiling of hypericin and related phytochemicals in Hypericum species using MALDI-HRMS imaging. Anal Bioanal Chem 407, 4779–4791 (2015). https://doi.org/10.1007/s00216-015-8682-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8682-6