Abstract
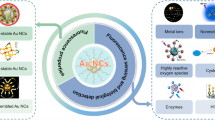
In this work, the chemiluminescence (CL) property of 5,10,15,20-tetrakis(4-carboxyphenyl)-porphyrin- and N-(4-aminobutyl)-N-ethylisoluminol-functionalized TiO2 nanoparticles (TiO2-TCPP-ABEI nanoluminophores) was studied for the first time. It was found that TiO2-TCPP-ABEI nanoluminophores exhibited excellent CL activity in the presence of H2O2. The CL mechanism has been proposed due to the reaction of ABEI with H2O2 and catalytic effect of TiO2 and TCPP. Furthermore, trisodium citrate-stabilized gold nanoparticles were observed to effectively quench the CL of TiO2-TCPP-ABEI due to CL resonance energy transfer (CRET). On this basis, a sensitive and selective CRET-based immunoassay was developed for the determination of copeptin by using TiO2-TCPP-ABEI nanoluminophores as both CL nanointerface and energy donor, and using cit-AuNPs as an effective energy receptor. The immunoassay exhibited a wide dynamic range from 5 × 10−12 to 1 × 10−9 g mL−1 with a low detection limit of 1.54 × 10−12 g mL−1, which was superior to previously reported CL-based immunoassays. It was successfully applied for the determination of copeptin in serum samples, which would provide a good practical perspective on the clinical diagnosis. This strategy may also be used for the detection of other antigens if corresponding antibodies are available.

Graphical abstract






Similar content being viewed by others
References
Bi S, Zhao TT, Luo BY. A graphene oxide platform for the assay of biomolecules based on chemiluminescence resonance energy transfer. Chem Commun. 2012;48:106–8.
Roy R, Hohng S, Ha T. A practical guide to single-molecule FRET. Nat Methods. 2008;5:507–16.
Schuler B, Lipman EA, Eaton WA. Probing the free-energy surface for protein folding with single-molecule fluorescence spectroscopy. Nature. 2002;419:743–7.
Pfleger KDG, Eidne KA. Illuminating insights into protein-protein interactions using bioluminescence resonance energy transfer (BRET). Nat Methods. 2006;3:165–74.
Yao HQ, Zhang Y, Xiao F, Xia ZY, Rao JH. Quantum dot/bioluminescence resonance energy transfer based highly sensitive detection of proteases. Angew Chem Int Ed. 2007;46:4346–9.
Li ZY, Lin ZF, Wu XY, Chen HT, Chai YQ, Yuan R. Highly efficient electrochemiluminescence resonance energy transfer system in one nanostructure: its application for ultrasensitive detection of microRNA in cancer cells. Anal Chem. 2017;89:6030–6.
Li P, Liu L, Xiao HB, Zhang W, Wang LL, Tang B. A new polymer nanoprobe based on chemiluminescence resonance energy transfer for ultrasensitive imaging of intrinsic superoxide anion in mice. J Am Chem Soc. 2016;138:2893–6.
Shuhendler AJ, Pu KY, Cui L, Uetrecht JP, Rao JH. Real-time imaging of oxidative and nitrosative stress in the liver of live animals for drug-toxicity testing. Nat Biotechnol. 2014;32:373–U240.
Hoffmann C, Gaietta G, Bunemann M, Adams SR, Oberdorff-Maass S, Behr B, et al. A FlAsH-based FRET approach to determine G protein - coupled receptor activation in living cells. Nat Methods. 2005;2:171–6.
Dehmelt L, Bastiaens PIH. Spatial organization of intracellular communication: insights from imaging. Nat Rev Mol Cell Biol. 2010;11:440–52.
Royer CA. Probing protein folding and conformational transitions with fluorescence. Chem Rev. 2006;106:1769–84.
Wang M, Hou W, Mi CC, Wang WX, Xu ZR, Teng HH, et al. Immunoassay of goat antihuman immunoglobulin G antibody based on luminescence resonance energy transfer between near-infrared responsive NaYF4:Yb, Er upconversion fluorescent nanoparticles and gold nanoparticles. Anal Chem. 2009;81:8783–9.
Qin GX, Zhao SL, Huang Y, Jiang J, Ye FG. Magnetic bead-sensing-platform-based chemiluminescence resonance energy transfer and its immunoassay application. Anal Chem. 2012;84:2708–12.
Huang XY, Ren JC. Nanomaterial-based chemiluminescence resonance energy transfer: a strategy to develop new analytical methods. TrAC Trends Anal Chem. 2012;40:77–89.
Du JJ, Yu CM, Pan DC, Li JM, Chen W, Yan M, et al. Quantum-dot-decorated robust transductable bioluminescent nanocapsules. J Am Chem Soc. 2010;132:12780–1.
Freeman R, Liu XQ, Willner I. Chemiluminescent and chemiluminescence resonance energy transfer (CRET) detection of DNA, metal ions, and aptamer-substrate complexes using hemin/G-quadruplexes and CdSe/ZnS quantum dots. J Am Chem Soc. 2011;133:11597–604.
Lee JS, Joung HA, Kim MG, Park CB. Graphene-based chemiluminescence resonance energy transfer for homogeneous immunoassay. ACS Nano. 2012;6:2978–83.
Zhang SS, Yan YM, Bi S. Design of molecular beacons as signaling probes for adenosine triphosphate detection in cancer cells based on chemiluminescence resonance energy transfer. Anal Chem. 2009;81:8695–701.
Bi S, Zhang JL, Hao SY, Ding CF, Zhang SS. Exponential amplification for chemiluminescence resonance energy transfer detection of microRNA in real samples based on a cross-catalyst strand-displacement network (vol 83, pg 3696, 2011). Anal Chem. 2011;83:4326.
Liu XQ, Freeman R, Golub E, Willner I. Chemiluminescence and chemiluminescence resonance energy transfer (CRET) aptamer sensors using catalytic hemin/G-quadruplexes. ACS Nano. 2011;5:7648–55.
Tian DY, Zhang HL, Chai Y, Cui H. Synthesis of N-(aminobutyl)-N-(ethylisoluminol) functionalized gold nanomaterials for chemiluminescent bio-probe. Chem Commun. 2011;47:4959–61.
Shu JN, Han ZL, Zheng TH, Du DX, Zou GZ, Cui H. Potential-resolved multicolor electrochemiluminescence of N-(4-aminobutyl)-N-thylisoluminol/tetra(4-carboxyphenyl)porphyrin/TiO2 nanoluminophores. Anal Chem. 2017;89:12636–40.
Morgenthaler NG, Struck J, Jochberger S, Dünser MW. Copeptin: clinical use of a new biomarker. Trends Endocrinol Metab. 2008;19:43–9.
Frens G. Controlled nucleation for regulation of particle-size in monodisperse gold suspensions. Nat Phys Sci. 1973;241:20–2.
Han ZL, Shu JN, Jiang QS, Cui H. Coreactant-free and label-free electrochemiluminescence immunosensor for copeptin based on luminescent immuno-gold nanoassemblies. Anal Chem. 2018;90:6064–70.
Barni F, Lewis SW, Berti A, Miskelly GM, Lago G. Forensic application of the luminol reaction as a presumptive test for latent blood detection. Talanta. 2007;72:896–913.
Dai PP, Yu T, Shi HW, Xu JJ, Chen HY. General strategy for enhancing electrochemiluminescence of semiconductor nanocrystals by hydrogen peroxide and potassium persulfate as dual coreactants. Anal Chem. 2015;87:12372–9.
Liu XY, Han ZL, Li F, Gao LF, Liang GL, Cui H. Highly chemiluminescent graphene oxide hybrids bifunctionalized by N-(aminobutyl)-N-(ethylisoluminol)/horseradish peroxidase and sensitive sensing of hydrogen peroxide. ACS Appl Mater Interfaces. 2015;7:18283–91.
Kuhn S, Hakanson U, Rogobete L, Sandoghdar V. Enhancement of single-molecule fluorescence using a gold nanoparticle as an optical nanoantenna. Phys Rev Lett. 2006;97:017402.
Anger P, Bharadwaj P, Novotny L. Enhancement and quenching of single-molecule fluorescence. Phys Rev Lett. 2006;96:113002.
Mayilo S, Kloster MA, Wunderlich M, Lutich A, Klar TA, Nichtl A, et al. Long-range fluorescence quenching by gold nanoparticles in a sandwich immunoassay for cardiac troponin. Nano Lett. 2009;9:4558–63.
Funding
The support of this research by the National Key Research and Development Program of China (Grant No. 2016YFA0201300) and the National Natural Science Foundation of China (Grant No. 21527807) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The research was approved by the Ethical Committee of the First Affiliated Hospital of Nanjing Medical University. All volunteers were informed of and agreed with the objectives of the study.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Published in the topical collection New Insights into Analytical Science in China with guest editors Lihua Zhang, Hua Cui, and Qiankun Zhuang.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 443 kb)
Rights and permissions
About this article
Cite this article
Shu, J., Han, Z. & Cui, H. Highly chemiluminescent TiO2/tetra(4-carboxyphenyl)porphyrin/N-(4-aminobutyl)-N-ethylisoluminol nanoluminophores for detection of heart disease biomarker copeptin based on chemiluminescence resonance energy transfer. Anal Bioanal Chem 411, 4175–4183 (2019). https://doi.org/10.1007/s00216-019-01821-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-01821-2