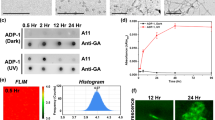
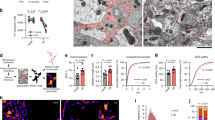
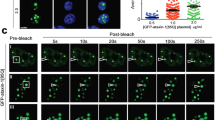
Abstract
Dipeptide repeats (DPRs) associated with C9orf72 repeat expansions perturb nucleocytoplasmic transport and are implicated in the pathogenesis of amyotrophic lateral sclerosis. We present a synthetic hydrogel platform that can be used to analyze aspects of the molecular interaction of dipeptide repeats and the phenylalanine-glycine (FG) phase of the nuclear pore complex (NPC). Hydrogel scaffolds composed of acrylamide and copolymerized with FG monomers are first formed to recapitulate key FG interactions found in the NPC. With labeled probes, we find evidence that toxic arginine-rich DPRs (poly-GR and poly-PR), but not the non-toxic poly-GP, target NPC hydrogel mimics and block selective entry of a key nuclear transport receptor, importin beta (Impβ). The ease with which these synthetic hydrogel mimics can be adjusted/altered makes them an invaluable tool to dissect complex molecular interactions that underlie cellular transport processes and their perturbation in disease.
Graphical abstract






Similar content being viewed by others
Data availability
Data is available from the authors on request.
References
Shin Y, Brangwynne CP. Liquid phase condensation in cell physiology and disease. Science. 2017;357(6357):eaaf4382.
Boeynaems S, Alberti S, Fawzi NL, Mittag T, Polymenidou M, Rousseau F, et al. Protein phase separation: a new phase in cell biology. Trends Cell Biol. 2018;28(6):420–35.
Alberti S, Dormann D. Liquid-liquid phase separation in disease. Annu Rev Genet. 2019;53:171–94.
Jo M, Lee S, Jeon YM, Kim S, Kwon Y, Kim HJ. The role of TDP-43 propagation in neurodegenerative diseases: integrating insights from clinical and experimental studies. Exp Mol Med. 2020;52(10):1652–62.
Ferrari R, Kapogiannis D, Huey ED, Momeni P. FTD and ALS: a tale of two diseases. Curr Alzheimer Res. 2011;8(3):273–94.
DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72(2):245–56.
Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72(2):257–68.
Renton AE, Chiò A, Traynor BJ. State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci. 2014;17(1):17–23.
Mori K, Weng SM, Arzberger T, May S, Rentzsch K, Kremmer E, et al. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science. 2013;339(6125):1335–8.
Ash PE, Bieniek KF, Gendron TF, Caulfield T, Lin WL, Dejesus-Hernandez M, et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron. 2013;77(4):639–46.
Zu T, Liu Y, Bañez-Coronel M, Reid T, Pletnikova O, Lewis J, et al. RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc Natl Acad Sci U S A. 2013;110(51):E4968–77.
Odeh HM, Shorter J. Arginine-rich dipeptide-repeat proteins as phase disruptors in C9-ALS/FTD. Emerg Top Life Sci. 2020;4(3):293–305.
Freibaum BD, Lu Y, Lopez-Gonzalez R, Kim NC, Almeida S, Lee KH, et al. GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature. 2015;525(7567):129–33.
Jovičić A, Mertens J, Boeynaems S, Bogaert E, Chai N, Yamada SB, et al. Modifiers of C9orf72 dipeptide repeat toxicity connect nucleocytoplasmic transport defects to FTD/ALS. Nat Neurosci. 2015;18(9):1226–9.
Boeynaems S, Bogaert E, Michiels E, Gijselinck I, Sieben A, Jovičić A, et al. Drosophila screen connects nuclear transport genes to DPR pathology in c9ALS/FTD. Sci Rep. 2016;6:20877.
Lee KH, Zhang P, Kim HJ, Mitrea DM, Sarkar M, Freibaum BD, et al. C9orf72 dipeptide repeats impair the assembly, dynamics, and function of membrane-less organelles. Cell. 2016;167(3):774–88.e17.
Lin Y, Mori E, Kato M, Xiang S, Wu L, Kwon I, et al. Toxic PR poly-dipeptides encoded by the C9orf72 repeat expansion target LC domain polymers. Cell. 2016;167(3):789–802.e12.
Boeynaems S, Bogaert E, Kovacs D, Konijnenberg A, Timmerman E, Volkov A, et al. Phase separation of C9orf72 dipeptide repeats perturbs stress granule dynamics. Mol Cell. 2017;65(6):1044–55.e5.
Frey S, Richter RP, Görlich D. FG-rich repeats of nuclear pore proteins form a three-dimensional meshwork with hydrogel-like properties. Science. 2006;314(5800):815–7.
Qamar S, Wang G, Randle SJ, Ruggeri FS, Varela JA, Lin JQ, et al. FUS phase separation is modulated by a molecular chaperone and methylation of arginine cation-π interactions. Cell. 2018;173(3):720–34.e15.
Wang J, Choi JM, Holehouse AS, Lee HO, Zhang X, Jahnel M, et al. A molecular grammar governing the driving forces for phase separation of prion-like RNA binding proteins. Cell. 2018;174(3):688–99.e16.
Bogaert E, Boeynaems S, Kato M, Guo L, Caulfield TR, Steyaert J, et al. Molecular dissection of FUS points at synergistic Effect of low-complexity domains in toxicity. Cell Rep. 2018;24(3):529–37.e4.
Shi KY, Mori E, Nizami ZF, Lin Y, Kato M, Xiang S, et al. Toxic PR(n) poly-dipeptides encoded by the C9orf72 repeat expansion block nuclear import and export. Proc Natl Acad Sci U S A. 2017;114(7):E1111–e7.
Hayes LR, Duan L, Bowen K, Kalab P, Rothstein JD. C9orf72 arginine-rich dipeptide repeat proteins disrupt karyopherin-mediated nuclear import. Elife. 2020;9:e51685.
Vanneste J, Vercruysse T, Boeynaems S, Sicart A, Van Damme P, Daelemans D, et al. C9orf72-generated poly-GR and poly-PR do not directly interfere with nucleocytoplasmic transport. Sci Rep. 2019;9(1):15728.
Friedman AK, Baker LA. Synthetic hydrogel mimics of the nuclear pore complex display selectivity dependent on FG-repeat concentration and electrostatics. Soft Matter. 2016;12(47):9477–84.
Bird SP, Baker LA. An abiotic analogue of the nuclear pore complex hydrogel. Biomacromolecules. 2011;12(9):3119–23.
Boeynaems S, De Decker M, Tompa P, Van Den Bosch L. Arginine-rich peptides can actively mediate liquid-liquid phase separation. Bio-protocol. 2017;7:e2525.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–5.
Burley SK, Petsko GA. Weakly polar interactions in proteins. Adv Protein Chem. 1988;39:125–89.
Maul GG, Deaven LL, Freed JJ, Campbell GLM, Becak W. Investigation of the determinants of nuclear-pore number. Cytogenet Cell Genet. 1980;26(2–4):175–90.
Garciasegura LM, Lafarga M, Berciano MT, Hernandez P, Andres MA. Distribution of nuclear-pores and chromatin organization in neurons and glial-cells of the rat cerebellar cortex. J Comp Neurol. 1989;290(3):440–50.
Frey S, Gorlich D. A saturated FG-repeat hydrogel can reproduce the permeability properties of nuclear pore complexes. Cell. 2007;130(3):512–23.
Boeynaems S, Holehouse AS, Weinhardt V, Kovacs D, Van Lindt J, Larabell C, et al. Spontaneous driving forces give rise to protein-RNA condensates with coexisting phases and complex material properties. Proc Natl Acad Sci U S A. 2019;116(16):7889–98.
Acknowledgements
The IUB Light Microscopy Imaging Center is gratefully acknowledged for access to confocal microscopy. S.B. acknowledges an EMBO Long Term Fellowship.
Funding
This work received funding from the National Science Foundation, DMR Biomaterials Award 0906843.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection celebrating ABCs 20th Anniversary.
Rights and permissions
About this article
Cite this article
Friedman, A.K., Boeynaems, S. & Baker, L.A. Synthetic hydrogel mimics of the nuclear pore complex for the study of nucleocytoplasmic transport defects in C9orf72 ALS/FTD. Anal Bioanal Chem 414, 525–532 (2022). https://doi.org/10.1007/s00216-021-03478-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03478-2