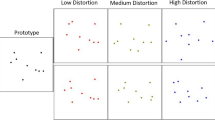
Abstract
Perceptual variability is often viewed as having multiple benefits in object learning and categorization. Despite the abundant results demonstrating benefits such as increased transfer of knowledge, the neural mechanisms underlying variability as well as the developmental trajectories of how variability precipitates changes to category boundaries are unknown. By manipulating an individual’s exposure to variability of novel, metrically organized categories during an fMRI-adaptation paradigm, we were able to assess the functional differences between similarity and variability in category learning and generalization across two time-points in development: adulthood (n = 14) and late childhood (n = 13). During this study, participants were repeatedly exposed to category members from different distributions. After a period of adaptation, a deviant stimulus that differed from the expected distribution was then presented. This deviant differed in either an invariant dimension (a feature that remained consistent throughout presentation was altered) or a similarity dimension (a feature that changed throughout exposure was changed in a new dimension). Our results can be summarized in three main findings: (1) Variability during exposure recruited the right fusiform gyrus to a greater extent than tight exposure. (2) Deviant items were generalized based on the exemplar distributions during exposure, although children only generalized items if provided variable exposure. (3) Variability influenced release to a greater extent in children than adults. These results are discussed in relation to the variability and category learning literature more broadly.







Similar content being viewed by others
Data availability
Data, stimuli, and other materials are available upon reasonable request from the authors. Interested parties can e-mail requests to the corresponding author.
Code availability
Code pertaining to the experimental protocol is also available upon reasonable request from the authors.
Notes
Of the included children, two children only contributed data from two functional runs due to excessive motion. For all children, this useable data was for separate categories and category structures, thus every participant contributed at least 1 useable run in each exposure condition. This resulted in 48 (24 tight and 24 variable) useable runs for children compared to 56 (28 tight and 28 variable) useable runs for adults.
To keep an equal number of variable and tight runs and an equal number of runs for each category, runs were grouped into pairs (Tight Flower and Far Alien; Tight Alien and Far Flower). Both runs in a pair were excluded if one of the runs had more than five spikes or they had a combined total of more than eight spikes. No adults had no motion spikes. Children had an average of 1.9 spikes on variable runs and an average of 1.6 spikes on tight runs.
Note that in many studies using similar category designs, the invariant feature is referred to as the rule (see Deng and Sloutsky, 2015 and 2016). However, because the categories were not explicitly taught, and because the design is ultimately agnostic to whether invariant deviants do or do not belong within the category, we believe “invariant deviants” to be a more accurate description of these items in this study.
References
Ashby FG, Valentin VV (2017) Multiple systems of perceptual category learning: theory and cognitive tests. In: Cohen H, Lefebvre C (eds) Handbook of categorization in cognitive science, 2nd edn. Elsevier, Cambridge, MA, pp 157–188
Ashby FG, Smith JD, Rosedahl LA (2019) Dissociations between rule-based and information-integration categorization are not caused by differences in task difficulty. Mem Cogn 48:1–12
Brainard DH, Vision S (1997) The psychophysics toolbox. Spat Vis 10:433–436
Bullmore ET, Brammer MJ, Rabe-Hesketh S, Curtis VA, Morris RG, Williams SCR et al (1999) Methods for diagnosis and treatment of stimulus-correlated motion in generic brain activation studies using fMRI. Hum Brain Mapp 7:38–48
Cohen AL, Nosofsky RM, Zaki SR (2001) Category variability, exemplar similarity, and perceptual classification. Mem Cogn 29(8):1165–1175
Conklin HM, Luciana M, Hooper CJ, Yarger RS (2007) Working memory performance in typically developing children and adolescents: behavioral evidence of protracted frontal lobe development. Dev Neuropsychol 31(1):103–128
Connolly JD, Goodale MA, Menon RS, Munoz DP (2002) Human fMRI evidence for the neural correlates of preparatory set. Nat Neurosci 5(12):1345–1352
Cowan N, Alloway TP (1997) The development of working memory. The development of memory in childhood. Psychology Press, pp 163–199
Dekker T, Mareschal D, Sereno MI, Johnson MH (2011) Dorsal and ventral stream activation and object recognition performance in school-age children. Neuroimage 57(3):659–670
Deng WS, Sloutsky VM (2015) The development of categorization: Effects of classification and inference training on category representation. Dev Psychol 51(3):392–405. https://doi.org/10.1037/a0038749
Deng WS, Sloutsky VM (2016) Selective attention, diffused attention, and the development of categorization. Cogn Psychol 91:24–62
Desimone R (1996) Neural mechanisms for visual memory and their role in attention. Proc Natl Acad Sci 93(24):13494–13499
Dhawale AK, Smith MA, Olveczky BP (2017) The role of variability in motor learning. Annu Rev Neurosci 40:479–498
Eidsvag SS, Austad M, Plante E, Asbjornsen AE (2015) Input variability facilitates unguided subcategory learning in adults. J Speech Lang Hear Res 58(3):826–839
Engle R, Carullo J, Collins K (1991) Individual differences in working memory for comprehension and following directions. J Educ Res 84(5):253–262
Estes WK, Burke CJ (1953) A theory of stimulus variability in learning. Psychol Rev 60(4):276–286
Fiser J, Aslin RN (2001) Unsupervised statistical learning of higher-order spatial structures from visual scenes. Psychol Sci 12(6):499–504
Folstein JR, Palmeri TJ, Gauthier I (2012) Category learning increases discriminability of relevant object dimensions in visual cortex. Cereb Cortex 23(4):814–823
Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, Gore JC (1999) Activation of the middle fusiform ‘face area’ increases with expertise in recognizing novel objects. Nat Neurosci 2(6):568–573
Golarai G, Ghahremani DG, Whitfield-Gabrieli S, Reiss A, Eberhardt JL, Gabrieli JD, Grill-Spector K (2007) Differential development of high-level visual cortex correlates with category-specific recognition memory. Nat Neurosci 10(4):512–522
Grill-Spector K, Malach R (2001) fMR-adaptation: a tool for studying the functional properties of human cortical neurons. Acta Physiol (Amst) 107(1–3):293–321
Grill-Spector K, Weiner KS (2014) The functional architecture of the ventral temporal cortex and its role in categorization. Nat Rev Neurosci 15(8):536–548
Grill-Spector K, Kourtzi Z, Kanwisher N (2001) The lateral occipital complex and its role in object recognition. Vis Res 41(10–11):1409–1422
Grill-Spector K, Knouf N, Kanwisher N (2004) The fusiform face area subserves face perception, not generic within-category identification. Nat Neurosci 7(5):555–562
Haist F, Adamo M, Wazny JH, Lee K, Stiles J (2013) The functional architecture for face-processing expertise: FMRI evidence of the developmental trajectory of the core and the extended face systems. Neuropsychologia 51(13):2893–2908
Harman KL, Humphrey GK (1999) Encoding ‘regular’ and ‘random’ sequences of views of novel three-dimensional objects. Perception 28(5):601–615
Hubel DH, Wiesel TN (1959) Receptive fields of single neurones in the cat’s striate cortex. J Physiol 148(3):574–591
James KH (2010) Sensori-motor experience leads to changes in visual processing in the developing brain. Dev Sci 13(2):279–288
James KH (2017) The importance of handwriting experience on the development of the literate brain. Curr Dir Psychol Sci 26(6):502–508
James KH, Engelhardt L (2012) The effects of handwriting experience on functional brain development in pre-literate children. Trends Neurosci Educ 1(1):32–42
James KH, Humphrey GK, Vilis T, Corrie B, Baddour R, Goodale MA (2002) “Active” and “passive” learning of three-dimensional object structure within an immersive virtual reality environment. Behav Res Methods Instrum Comput 34(3):383–390
James KH, Jones SS, Smith LB, Swain SN (2014) Young children’s self-generated object views and object recognition. J Cogn Dev 15(3):393–401
Johnson MH (2001) Functional brain development in humans. Nat Rev Neurosci 2(7):475–483
Joseph JE, Zhu X, Gundran A, Clark JD, Ruble L, Glaser P, Bhatt RS (2015) Typical and atypical neurodevelopment for face specialization: an FMRI study. J Autism Dev Disord 45(6):1725–1741
Kadosh KC, Johnson MH, Henson RN, Dick F, Blakemore SJ (2013) Differential face-network adaptation in children, adolescents and adults. Neuroimage 69:11–20
Kanwisher N, McDermott J, Chun MM (1997) The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci 17(11):4302–4311
Konrad K, Neufang S, Thiel CM, Specht K, Hanisch C, Fan J, Herpertz-Dahlmann B, Fink GR (2005) Development of attentional networks: an fMRI study with children and adults. Neuroimage 28(2):429–439
Li JX, James KH (2016) Handwriting generates variable visual output to facilitate symbol learning. J Exp Psychol Gen 145(3):298–313
Manning C, Jones PR, Dekker TM, Pellicano E (2018) Psychophysics with children: investigating the effects of attentional lapses on threshold estimates. Atten Percept Psychophys 80(5):1311–1324
Medin DL, Goldstone RL, Gentner D (1993) Respects for similarity. Psychol Rev 100(2):254–278
Nishimura M, Scherf S, Behrmann M (2009) Development of object recognition in humans. F1000 Biol Rep 1:56
Passarotti AM, Paul BM, Bussiere JR, Buxton RB, Wong EC, Stiles J (2003) The development of face and location processing: an fMRI study. Dev Sci 6(1):100–117
Pelli DG, Vision S (1997) The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10:437–442
Perone S, Molitor SJ, Buss AT, Spencer JP, Samuelson LK (2015) Enhancing the executive functions of 3-year-olds in the dimensional change card sort task. Child Dev 86(3):812–827
Perone S, Plebanek DJ, Lorenz MG, Spencer JP, Samuelson LK (2019) Empirical tests of a brain-based model of executive function development. Child Dev 90(1):210–226
Perry LK, Samuelson LK, Malloy LM, Schiffer RN (2010) Learn locally, think globally: exemplar variability supports higher-order generalization and word learning. Psychol Sci 21(12):1894–1902
Plebanek DJ, James KH (under review) Variability among members expand category spaces but do not extract category rules
Plebanek DJ, Sloutsky VM (2019) Selective attention, filtering, and the development of working memory. Dev Sci 22(1):e12727
Posner MI, Keele SW (1968) On the genesis of abstract ideas. J Exp Psychol 77(3 Pt 1):353–363
Quinn PC, Eimas PD, Rosenkrantz SL (1993) Evidence for representations of perceptually similar natural categories by 3-month-old and 4-month-old infants. Perception 22(4):463–475
Rabi R, Miles SJ, Minda JP (2015) Learning categories via rules and similarity: Comparing adults and children. J Exp Child Psychol 131:149–169
Rips L (1989) Similarity, typicality, and categorization. Similarity and analogical reasoning. Cambridge University Press, pp 21–59
Saffran JR, Aslin RN, Newport EL (1996) Statistical learning by 8-month-old infants. Science 274(5294):1926–1928
Scherf KS, Luna B, Avidan G, Behrmann M (2011) “What” precedes “which”: developmental neural tuning in face-and place-related cortex. Cereb Cortex 21(9):1963–1980
Scherf S, Thomas K, Doyle C, Behrmann M (2014) Emerging structure–function relations in the developing face processing system. Cereb Cortex 24(11):2964–2980
Slone LK, Smith LB, Yu C (2019) Self-generated variability in object images predicts vocabulary growth. Dev Sci 22:12816
Sloutsky VM (2010) From perceptual categories to concepts: what develops? Cogn Sci 34(7):1244–1286
Sloutsky VM (2015) The development of categorization: Effects of classification and inference training on category representation. Dev Psychol 51(3):392–405
Smith LB (1989) A model of perceptual classification in children and adults. Psychol Rev 96(1):125
Smith LB, Kemler DG (1977) Developmental trends in free classification: evidence for a new conceptualization of perceptual development. J Exp Child Psychol 24(2):279–298
Smith LB, Thelen E (2003) Development as a dynamic system. Trends Cogn Sci 7(8):343–348
Talairach J, Tournoux P (1988) Co-planar stereotaxic atlas of the human brain. 3-dimensional proportional system: an approach to cerebral imaging. Thieme, New York
Tarr MJ, Gauthier I (2000) FFA: a flexible fusiform area for subordinate-level visual processing automatized by expertise. Nat Neurosci 3(8):764–769
Twomey KE, Lush L, Pearce R, Horst JS (2014) Visual variability affects early verb learning. Br J Dev Psychol 32(3):359–366
Turk-Browne NB, Scholl BJ, Chun MM, Johnson MK (2009) Neural evidence of statistical learning: efficient detection of visual regularities without awareness. J Cogn Neurosci 21(10):1934–1945
Vernet M, Quentin R, Chanes L, Mitsumasu A, Valero-Cabré A (2014) Frontal eye field, where art thou? Anatomy, function, and non-invasive manipulation of frontal regions involved in eye movements and associated cognitive operations. Front Integr Neurosci 8:1–66
Vinci-Booher S, James TW, James KH (2020) Visual-motor contingency during symbol production contributes to the development of the neural systems supporting symbol perception and concurrent gains in symbol recognition. Neuroimage 227:117554
Yang Q, Bucci MP, Kapoula Z (2002) The latency of saccades, vergence, and combined eye movements in children and in adults. Invest Ophthalmol Vis Sci 43(9):2939–2949
Zemlock D, Vinci-Booher S, James KH (2018) Visual–motor symbol production facilitates letter recognition in young children. Read Writ 31(6):1255–1271
Acknowledgements
We thank members of the Imaging Research Facility at Indiana University for conversations and suggestions concerning study procedures and the CAN Lab research assistants involved in this project. This project was supported by National Institute of Health 2 T32 Grant # HD 007475-21 and by the Indiana University Office of the Vice President for Research Emerging Area of Research Initiative, Learning: Brains, Machines, and Children. No funding sources were involved in the study design, analysis, or interpretation of the data, in the writing of this article, or in the decision to submit this article for publication.
Funding
This project was supported by National Institute of Health 2 T32 Grant # HD 007475-21 and by the Indiana University Office of the Vice President for Research Emerging Area of Research Initiative, Learning: Brains, Machines, and Children.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception, design, data analysis, and writing. Material preparation and data collection were performed by DP. KJ is the PI of the laboratory, supervised the other author and funded the project. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to report.
Additional information
Communicated by Winston D Byblow.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1
Appendix 1
The metric changes were as follows: Within the alien similarity space, the metric changes were as follows: the eyestalk changed 0.08 inches per step, the surface area changed by adjusting the width of the body approximately 0.08 inches and the height approximately 0.07 inches, the arms changed 5° per step. Within the flower similarity space, the stems changed the zigzag function in adobe illustrator 5%, the petals were rounded 5°, the gradient of the center changed 5%. The metric nature of our categories resulted in numerical differences in our exposure types. The tight variability consisted of a similarity space that could only vary a maximum of one value per each of the three dimensions in the similarity space. Thus, potential values included only values 8 through 10 on each of the 3 dimensions. The tight exposure included a distribution of stimuli that varied from the central stimulus (stimulus 9-9-9) an average of 2.05 steps, with the average of each dimension in the similarity space being (9.01, 8.89, 9.06). For the variable space, the similarity space could vary a maximum of five values per each of the three dimensions in the similarity space. Therefore, potential values included 4–14 on each dimension. This distribution varied from the central stimulus an average of 8.25 steps, and the average of the stimulus space was (8.17, 8.52, 8.96).
Rights and permissions
About this article
Cite this article
Plebanek, D.J., James, K.H. Category structure guides the formation of neural representations. Exp Brain Res 239, 1667–1684 (2021). https://doi.org/10.1007/s00221-021-06088-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-021-06088-7