Abstract
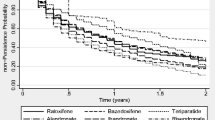
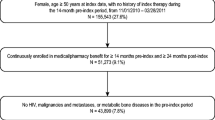
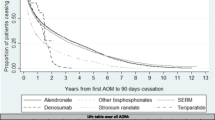
This study assessed persistence and compliance with anti-osteoporosis therapies, and associations between compliance and clinical outcomes (fracture, fracture-related hospitalization and death), in Hungarian women with postmenopausal osteoporosis. The study used the Hungarian National Health Insurance Fund Administration database and included women with PMO aged at least 50 years, for whom a prescription for anti-osteoporosis medication had been filled between 1 January 2004 and 31 December 2013 (index event). Persistence (prescription refilled within 8 weeks of the end of the previous supply) was evaluated over 2 years; good compliance (medication possession ratio ≥ 80 %) was evaluated at 1 year. Associations between compliance and clinical outcomes (data collected for up to 6 years) were assessed with adjustment for baseline covariates. A total of 296,300 women met the inclusion criteria (524,798 index events). Persistence and compliance were higher for less frequent and parenteral therapies (1- and 2-year persistence: half-yearly [parenteral] vs. daily/weekly/monthly [oral and parenteral], 81 and 38 % vs. 21–34 and 10–18 %, respectively; parenteral vs. oral, 75 and 36 % vs. 32 and 16 %; good compliance: half-yearly vs. daily/weekly/monthly, 70 vs. 24–39 %; parenteral vs. oral 78 vs. 36 %). Good compliance significantly reduced the risks of fracture, fracture-related hospitalization and death (relative risk vs. non-compliance [95 % confidence interval]: 0.77 [0.70–0.84], 0.72 [0.62–0.85] and 0.57 [0.51–0.64], respectively; P < 0.01). Improving compliance through long-interval parenteral therapies may result in clinical benefits for patients.


Similar content being viewed by others
References
Consensus development conference (1993) Diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med 94:646–650
NHIFA MaCD (2009) Osteoporosis miatti csonttörések primer és szekunder prevenciója, “Finanszírozási protokoll háttéranyaga”. Országos Egészségbiztosítási Pénztár Elemzési, Orvosszakértői és Szakmai Ellenőrzési Főosztály. (Primary and secondary prevention of osteoporosis fractures “Financing-protocol background material”. http://site.oep.hu/prot/Az_osteoporosis_terapia_finanszirozasi_protokolljainak_hatteranyaga2.pdf. Accessed Oct 2015
Pentek M, Horvath C, Boncz I, Falusi Z, Toth E, Sebestyen A, Majer I, Brodszky V, Gulacsi L (2008) Epidemiology of osteoporosis related fractures in Hungary from the nationwide health insurance database, 1999-2003. Osteoporos Int 19(2):243–249. doi:10.1007/s00198-007-0453-6
Delmas PD (2002) Treatment of postmenopausal osteoporosis. Lancet 359(9322):2018–2026. doi:10.1016/S0140-6736(02)08827-X
Kanis JA, Johnell O (2005) Requirements for DXA for the management of osteoporosis in Europe. Osteoporos Int 16(3):229–238. doi:10.1007/s00198-004-1811-2
Silverman SL, Gold DT (2008) Compliance and persistence with osteoporosis therapies. Curr Rheumatol Rep 10(2):118–122
Cramer JA, Amonkar MM, Hebborn A, Altman R (2005) Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin 21(9):1453–1460. doi:10.1185/030079905X61875
Huybrechts KF, Ishak KJ, Caro JJ (2006) Assessment of compliance with osteoporosis treatment and its consequences in a managed care population. Bone 38(6):922–928. doi:10.1016/j.bone.2005.10.022
Solomon DH, Avorn J, Katz JN, Finkelstein JS, Arnold M, Polinski JM, Brookhart MA (2005) Compliance with osteoporosis medications. Arch Int Med 165(20):2414–2419. doi:10.1001/archinte.165.20.2414
McCombs JS, Thiebaud P, McLaughlin-Miley C, Shi J (2004) Compliance with drug therapies for the treatment and prevention of osteoporosis. Maturitas 48(3):271–287. doi:10.1016/j.maturitas.2004.02.005
Lo JC, Pressman AR, Omar MA, Ettinger B (2006) Persistence with weekly alendronate therapy among postmenopausal women. Osteoporos Int 17(6):922–928. doi:10.1007/s00198-006-0085-2
Hadji P, Claus V, Ziller V, Intorcia M, Kostev K, Steinle T (2012) GRAND: the German retrospective cohort analysis on compliance and persistence and the associated risk of fractures in osteoporotic women treated with oral bisphosphonates. Osteoporos Int 23(1):223–231. doi:10.1007/s00198-011-1535-z
Karlsson L, Lundkvist J, Psachoulia E, Intorcia M, Strom O (2015) Persistence with denosumab and persistence with oral bisphosphonates for the treatment of postmenopausal osteoporosis: a retrospective, observational study, and a meta-analysis. Osteoporos Int 26(10):2401–2411. doi:10.1007/s00198-015-3253-4
Siris ES, Selby PL, Saag KG, Borgstrom F, Herings RM, Silverman SL (2009) Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am J Med 122(2 Suppl):S3–S13. doi:10.1016/j.amjmed.2008.12.002
Melton LJ 3rd, Gabriel SE, Crowson CS, Tosteson AN, Johnell O, Kanis JA (2003) Cost-equivalence of different osteoporotic fractures. Osteoporos Int 14(5):383–388. doi:10.1007/s00198-003-1385-4
McCloskey E, De Takats D, Orgee J et al (2013) Characteristics associated with non-persistence during daily therapy. Experience from the placebo wing of a community-based clinical trial. J Bone Min Res 20(1):2025
Lamberg L (2000) Sleep disorders, often unrecognized, complicate many physical illnesses. JAMA 284(17):2173–2175
Eraker SA, Kirscht JP, Becker MH (1984) Understanding and improving patient compliance. Ann Int Med 100(2):258–268
Claxton AJ, Cramer J, Pierce C (2001) A systematic review of the associations between dose regimens and medication compliance. Clin Ther 23(8):1296–1310
Diaz Curiel M, Carrasco de la Pena JL, Honorato Perez J, Perez Cano R, Rapado A, Ruiz Martinez I (1997) Study of bone mineral density in lumbar spine and femoral neck in a Spanish population. Multicentre Research Project on Osteoporosis. Osteoporos Int 7(1):59–64
Unson CG, Siccion E, Gaztambide J, Gaztambide S, Mahoney Trella P, Prestwood K (2003) Nonadherence and osteoporosis treatment preferences of older women: a qualitative study. J Women’s Health 12(10):1037–1045. doi:10.1089/154099903322643965
Nevitt MC, Ettinger B, Black DM, Stone K, Jamal SA, Ensrud K, Segal M, Genant HK, Cummings SR (1998) The association of radiographically detected vertebral fractures with back pain and function: a prospective study. Ann Int Med 128(10):793–800
van den Boogaard CH, Breekveldt-Postma NS, Borggreve SE, Goettsch WG, Herings RM (2006) Persistent bisphosphonate use and the risk of osteoporotic fractures in clinical practice: a database analysis study. Curr Med Res Opin 22(9):1757–1764. doi:10.1185/030079906X132370
Lakatos PT (2013) Persistence & compliance to treatment for osteoporosis in postmenopausal women in Hungary: a retrospective cohort study. Value Health 16:A567–A568
Hadji P, Papaioannou N, Gielen E, Feudjo Tepie M, Zhang E, Frieling I, Geusens P, Makras P, Resch H, Moller G, Kalouche-Khalil L, Fahrleitner-Pammer A (2015) Persistence, adherence, and medication-taking behavior in women with postmenopausal osteoporosis receiving denosumab in routine practice in Germany, Austria, Greece, and Belgium: 12 month results from a European non-interventional study. Osteoporos Int 26:2479–2489. doi:10.1007/s00198-015-3164-4
Silverman SL, Siris E, Kendler DL, Belazi D, Brown JP, Gold DT, Lewiecki EM, Papaioannou A, Simonelli C, Ferreira I, Balasubramanian A, Dakin P, Ho P, Siddhanti S, Stolshek B, Recknor C (2015) Persistence at 12 months with denosumab in postmenopausal women with osteoporosis: interim results from a prospective observational study. Osteoporos Int 26:361–372
Hernlund E, Svedbom A, Ivergard M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jonsson B, Kanis JA (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8:136. doi:10.1007/s11657-013-0136-1
Siris ES, Harris ST, Rosen CJ, Barr CE, Arvesen JN, Abbott TA, Silverman S (2006) Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc 81(8):1013–1022. doi:10.4065/81.8.1013
Hoer A, Seidlitz C, Gothe H, Schiffhorst G, Olson M, Hadji P, Haussler B (2009) Influence on persistence and adherence with oral bisphosphonates on fracture rates in osteoporosis. Patient Prefer Adher 3:25–30
LaFleur J, McAdam-Marx C, Kirkness C, Brixner DI (2008) Clinical risk factors for fracture in postmenopausal osteoporotic women: a review of the recent literature. Ann Pharmacother 42(3):375–386. doi:10.1345/aph.1K203
National Health Insurance Fund of Hungary (Hungarian acronym: OEP) http://www.oep.hu. Accessed Nov 2014
Black DM, Reid IR, Boonen S, Bucci-Rechtweg C, Cauley JA, Cosman F, Cummings SR, Hue TF, Lippuner K, Lakatos P, Leung PC, Man Z, Martinez RL, Tan M, Ruzycky ME, Su G, Eastell R (2012) The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 27(2):243–254. doi:10.1002/jbmr.1494
Reid IR, Black DM, Eastell R, Bucci-Rechtweg C, Su G, Hue TF, Mesenbrink P, Lyles KW, Boonen S, Trial HPF, Committees HRFTS (2013) Reduction in the risk of clinical fractures after a single dose of zoledronic Acid 5 milligrams. J Clin Endocrinol Metab 98(2):557–563. doi:10.1210/jc.2012-2868
Eli Lilly (2014) Forsteo (teriparatide) Summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000425/WC500027994.pdf. Accessed Sep 2015
Brown JP, Roux C, Torring O, Ho PR, Beck Jensen JE, Gilchrist N, Recknor C, Austin M, Wang A, Grauer A, Wagman RB (2013) Discontinuation of denosumab and associated fracture incidence: analysis from the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial. J Bone Miner Res 28(4):746–752. doi:10.1002/jbmr.1808
Confavreux CB, Canoui-Poitrine F, Schott AM, Ambrosi V, Tainturier V, Chapurlat RD (2012) Persistence at 1 year of oral antiosteoporotic drugs: a prospective study in a comprehensive health insurance database. Eur J Endocrinol 166(4):735–741. doi:10.1530/EJE-11-0959
Landfeldt E, Strom O, Robbins S, Borgstrom F (2012) Adherence to treatment of primary osteoporosis and its association to fractures–the Swedish Adherence Register Analysis (SARA). Osteoporos Int 23(2):433–443. doi:10.1007/s00198-011-1549-6
Briesacher BA, Andrade SE, Yood RA, Kahler KH (2007) Consequences of poor compliance with bisphosphonates. Bone 41(5):882–887. doi:10.1016/j.bone.2007.07.009
Penning-van Beest FJ, Erkens JA, Olson M, Herings RM (2008) Loss of treatment benefit due to low compliance with bisphosphonate therapy. Osteoporos Int 19(4):511–517. doi:10.1007/s00198-007-0466-1
Bliuc D, Nguyen ND, Nguyen TV, Eisman JA, Center JR (2013) Compound risk of high mortality following osteoporotic fracture and refracture in elderly women and men. J Bone Miner Res 28(11):2317–2324. doi:10.1002/jbmr.1968
International Osteoporosis Foundation Fracture risk map. http://www.iofbonehealth.org/facts-and-statistics/frax-map. Accessed Oct 2015
van Mierlo T, Fournier R, Ingham M (2015) Targeting medication non-adherence behavior in selected autoimmune diseases: a systematic approach to digital health program development. PLoS One 10(6):e0129364. doi:10.1371/journal.pone.0129364
Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M (2007) A checklist for medication compliance and persistence studies using retrospective databases. Value Health 10(1):3–12. doi:10.1111/j.1524-4733.2006.00139.x
Li L, Roddam A, Ferguson S, Feudjo-Tepie M, Taylor A, Jick S (2014) Switch patterns of osteoporosis medication and its impact on persistence among postmenopausal women in the U.K. General Practice Research Database. Menopause 21(10):1106–1113. doi:10.1097/GME.0000000000000214
Li L, Roddam A, Gitlin M, Taylor A, Shepherd S, Shearer A, Jick S (2012) Persistence with osteoporosis medications among postmenopausal women in the UK General Practice Research Database. Menopause 19(1):33–40. doi:10.1097/gme.0b013e318221bacd
Acknowledgments
Statistical support was provided by Pál Rakonczai and Tamás Balázs of HealthWare Consulting Ltd (funded by Amgen [Europe] GmbH). Editing support was provided by Claire Desborough, of Amgen (Europe) GmbH, and Oxford PharmaGenesis (funded by Amgen [Europe] GmbH).
Author Contributors
PL designed the study, interpreted the data and prepared early drafts of the paper. PL is guarantor. IT and IM interpreted the data. ET designed the study, and acquired, analysed and interpreted the data. CZ and ZL acquired, analysed and interpreted the data. EP and MI designed the study and interpreted the data. All authors revised the paper critically for intellectual content and approved the final version. All authors agree to be accountable for the work and to ensure that any questions relating to the accuracy and integrity of the paper are investigated and properly revised.
Funding
The study was funded by Amgen (Europe) GmbH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
PL, IT and IM have received consulting, research and speaker fees and grants from many companies with drugs for bone diseases, including Amgen. ET was an employee of Healthware Tanácsadó Kft when the study was conducted and has been in employment at UCB Biopharma Sprl as a Senior Manager Health Economics & Outcomes Research from December 2013. ZL and CZ are/were employees of Healthware Ltd and conducted this research under contract to Amgen. MI and EP are employees and shareholders of Amgen.
Human and Animal Rights and Informed Consent
For this type of study formal consent is not required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lakatos, P., Takács, I., Marton, I. et al. A Retrospective Longitudinal Database Study of Persistence and Compliance with Treatment of Osteoporosis in Hungary. Calcif Tissue Int 98, 215–225 (2016). https://doi.org/10.1007/s00223-015-0082-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-015-0082-6