Abstract
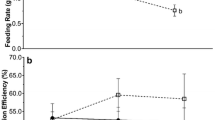
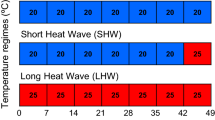
Highly dynamic environments such as estuaries are home to organisms accustomed to wide fluctuations in environmental conditions. However, estuarine temperature and salinity conditions are expected to shift with climate change, potentially altering plant and animal physiology and consequently their ecological interactions. Phyllaplysia taylori, a sea hare that lives exclusively in nearshore eelgrass beds in the Eastern Pacific Ocean, is a positive ecological interactor with eelgrass by increasing eelgrass productivity through grazing removal of photosynthesis-blocking epiphytes. The central aim of our study is to understand how increasing temperature and salinity are likely to alter that ecological interaction. First, we determined salinity thresholds for survival of P. taylori at 20 °C (typical summer temperature) for 2 weeks, and found that significant mortality occurs at salinity below 25 ppt. Then, we determined respiration rate, grazing rate, and defecation rate of P. taylori following a crossed 2-week acclimation at typical summer low- and high temperatures (18 and 22 °C) and salinities (27 and 33 ppt). P. taylori respiration and grazing rates were elevated under low salinity and high temperature. To determine how P. taylori responds to very warm and extreme summer temperatures, we measured respiration rates at higher temperatures (26 °C—very warm summer and 30 °C—heat shock) and feeding rates following exposure to the 30 °C heat shock. Irrespective of acclimation salinity, P. taylori acclimated to 18 °C were more sensitive to heat shock, as they had a larger increase in respiration rate at 30 °C, and had reduced feeding rates following the 30 °C exposure, whereas there was no reduction in feeding rate in 22 °C acclimated specimens. This study provides the first data on the salinity and temperature sensitivity and metabolic physiology of P. taylori with relevance to their trophic position in the context of eelgrass ecosystems.






Similar content being viewed by others
References
Baojun T, Baozhong L, Hongsheng Y, Jianhai X (2005) Oxygen consumption and ammonia–N excretion of Meretrix meretrix in different temperature and salinity. Chin J Oceanol Limnol 23:469–474
Bedford JJ (1972) Osmoregulation in Melanopsis trifasciata. II. The osmotic pressure and the principal ions of the hemocoelic fluid. Physiol Zool 45:261–269
Beeman R (1963) Variation and Synonymy of Phyllaplysia in the Northeastern Pacific. Veliger 6:43–47
Beeman R (1966) The biology of reproduction in Phyllaplysia taylori Dall, 1900. Doctor of Philosophy, Stanford University, Stanford
Beeman RD (1970) An autoradiographic study of sperm exchange and storage in a sea hare, Phyllaplysia taylori, a hermaphroditic gastropod (Opisthobranchia: Anaspidea). J Exp Zool Part Ecol Genet Physiol 175:125–132
Beiras R, Camacho AP, Albentosa M (1995) Short-term and long-term alterations in the energy budget of young oyster Ostrea edulis L. in response to temperature change. J Exp Mar Biol Ecol 186:221–236. https://doi.org/10.1016/0022-0981(94)00159-B
Boyer KE, Wyllie-Echeverria S (2010) Eelgrass conservation and restoration in San Francisco Bay: opportunities and constraints. San Francisco Bay Subtidal Habitat Goals Project, pp 83
Brodersen KE, Lichtenberg M, Paz L-C, Kühl M (2015) Epiphyte-cover on seagrass (Zostera marina L.) leaves impedes plant performance and radial O2 loss from the below-ground tissue. Front Mar Sci 2:58
Burg MB, Kwon ED, Kültz D (1996) Osmotic regulation of gene expression. FASEB J 10:1598–1606
Cayan DR, Peterson DH (1993) Spring climate and salinity in the San Francisco Bay estuary. Water Resour Res 29:293–303
Chang AL, Brown CW, Crooks JA, Ruiz GM (2018) Dry and wet periods drive rapid shifts in community assembly in an estuarine ecosystem. Glob Change Biol 24:e627–e642
Clark MS, Thorne MAS, King M, Hipperson H, Hoffman JI, Peck LS (2018) Life in the intertidal: cellular responses, methylation and epigenetics. Funct Ecol 32:1982–1994
Cloern JE (2019) Patterns, pace, and processes of water-quality variability in a long-studied estuary. Limnol Oceanogr 64:S192–S208
Cloern JE, Knowles N, Brown LR, Cayan D, Dettinger MD, Morgan TL, Schoellhamer DH, Stacey MT, van der Wegen M, Wagner RW (2011) Projected evolution of California’s San Francisco Bay-Delta-River system in a century of climate change. PLoS One 6:e24465
Cloern JE, Jassby AD, Schraga TS, Nejad E, Martin C (2017) Ecosystem variability along the estuarine salinity gradient: examples from long-term study of San Francisco Bay. Limnol Oceanogr 62:S272–S291
Deaton L (2008) Osmotic and ionic regulation in molluscs. Osmotic Ion Regul Cells Anim. https://doi.org/10.1201/9780849380525.ch4
Duffy JE, Macdonald KS, Rhode JM, Parker JD (2001) Grazer diversity, functional redundancy, and productivity in seagrass beds: an experimental test. Ecology 82:2417–2434. https://doi.org/10.1890/0012-9658(2001)082%5b2417:gdfrap%5d2.0.co;2
Duffy JE, Paul Richardson J, Canuel EA (2003) Grazer diversity effects on ecosystem functioning in seagrass beds. Ecol Lett 6:637–645
Edwards M, Richardson AJ (2004) Impact of climate change on marine pelagic phenology and trophic mismatch. Nature 430:881
Fernández-Reiriz MJ, Navarro JM, Labarta U (2005) Enzymatic and feeding behaviour of Argopecten purpuratus under variation in salinity and food supply. Comp Biochem Physiol A Mol Integr Physiol 141:153–163
Hammer KJ, Borum J, Hasler-Sheetal H, Shields EC, Sand-Jensen K, Moore KA (2018) High temperatures cause reduced growth, plant death and metabolic changes in eelgrass Zostera marina. Mar Ecol Prog Ser 604:121–132
Harada AE, Burton RS (2019) Ecologically relevant temperature ramping rates enhance the protective heat shock response in an intertidal ectotherm. Physiol Biochem Zool 92:152–162
Hearn CJ, Largier JL (1997) The summer buoyancy dynamics of a shallow Mediterranean estuary and some effects of changing bathymetry: Tomales Bay, California. Estuar Coast Shelf Sci 45:497–506
Hovel KA, Warneke AM, Virtue-Hilborn SP, Sanchez AE (2016) Mesopredator foraging success in eelgrass (Zostera marina L.): relative effects of epiphytes, shoot density, and prey abundance. J Exp Mar Biol Ecol 474:142–147
Hughes AR, Best RJ, Stachowicz JJ (2010) Genotypic diversity and grazer identity interactively influence seagrass and grazer biomass. Mar Ecol Prog Ser 403:43–51
Hughes BB, Lummis SC, Anderson SC, Kroeker KJ (2018) Unexpected resilience of a seagrass system exposed to global stressors. Glob Change Biol 24:224–234
Idrisi N, Barimo J, Hudder A, Capo T, Walsh P (2006) Rates of nitrogen excretion and oxygen consumption in the California Sea hare, Aplysia californica. Bull Mar Sci 79:231–237
Jaschinski S, Sommer U (2008) Top-down and bottom-up control in an eelgrass–epiphyte system. Oikos 117:754–762
Jobling M, Davies PS (1980) Effects of feeding on metabolic rate, and the specific dynamic action in plaice, Pleuronectes platessa L. J Fish Biol 16:629–638
Kadakkuzha BM, Akhmedov K, Capo TR, Carvalloza AC, Fallahi M, Puthanveettil SV (2013) Age-associated bidirectional modulation of gene expression in single identified R15 neuron of Aplysia. BMC Genom 14:880
Keeton RA, Runge SW, Moran WM (2004) Constitutive apical membrane recycling in Aplysia enterocytes. J Exp Zool A Comp Exp Biol 301:857–866
Khlebovich V (2017) Acclimation of animal organisms: basic theory and applied aspects. Biol Bull Rev 7:279–286
Kimmerer W (2002) Effects of freshwater flow on abundance of estuarine organisms: physical effects or trophic linkages? Mar Ecol Prog Ser 243:39–55
Kimmerer WJ (2004) Open water processes of the San Francisco Estuary [electronic resource]. John Muir Institute of the Environment, Davis
Kültz D (2001) Evolution of osmosensory MAP kinase signaling pathways. Am Zool 41:743–757
Kültz D (2005) Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol 67:225–257. https://doi.org/10.1146/annurev.physiol.67.040403.103635
Lewis JT, Boyer KE (2014) Grazer functional roles, induced defenses, and indirect interactions: implications for eelgrass restoration in San Francisco Bay. Diversity 14242818(6):751–770
Li C, Kong FN, Sun PP, Bi GQ, Li N, Mao YX, Sun J (2018) Genome-wide identification and expression pattern analysis under abiotic stress of mitogen-activated protein kinase genes in Pyropia yezoensis. J Appl Phycol 30:2561–2572
Lockwood APM (1976) Physiological adaptation to life in estuaries. In: Newell RC (ed) Adaptation to Environment. Butterworth-Heinemann, pp 315–392
Lockwood AM, Sheader M, Williams JA (1996) Life in estuaries, salt marshes, lagoons, and coastal waters. In: Summerhayes CP, Thorpe SA (eds) Oceanography: an illustrated guide. CRC Press, Taylor & Francis Group, Boca Raton, pp 244–258
Lockwood BL, Connor KM, Gracey AY (2015) The environmentally tuned transcriptomes of Mytilus mussels. J Exp Biol 218:1822–1833. https://doi.org/10.1242/jeb.118190
Low JS, Chew LL, Ng CC, Goh HC, Lehette P, Chong VC (2018) Heat shock response and metabolic stress in the tropical estuarine copepod Pseudodiaptomus annandalei converge at its upper thermal optimum. J Therm Biol 74:14–22
Martillotti AW, Tsai P-S (2018) An adipokinetic hormone acts as a volume regulator in the intertidal gastropod mollusk. Aplysia californica. Front Endocrinol 9:493
Miller NA, Chen X, Stillman JH (2014) Metabolic physiology of the invasive clam, Potamocorbula amurensis: the interactive role of temperature, salinity, and food availability. PLoS One 9:e91064. https://doi.org/10.1371/journal.pone.0091064
Mochida K, Hano T, Onduka T, Ito K, Yoshida G (2019) Physiological responses of eelgrass (Zostera marina) to ambient stresses such as herbicide, insufficient light, and high water temperature. Aquat Toxicol 208:20–28
Neckles HA, Wetzel RL, Orth RJ (1993) Relative effects of nutrient enrichment and grazing on epiphyte–macrophyte (Zostera marina L.) dynamics. Oecologia 93:285–295
Newell RC (1976) Adaptations to intertidal life. In: Newell RC (ed) Adaptation to Environment. Butterworth-Heinemann, pp 1–82
Newell R, Branch G (1980) The influence of temperature on the maintenance of metabolic energy balance in marine invertebrates. In: Advances in marine biology, vol 17. Academic Press, pp 329–396
Padilla-Ramírez S, Díaz F, Re AD, Galindo-Sanchez CE, Sanchez-Lizarraga AL, Nuñez-Moreno LA, Moreno-Sierra D, Paschke K, Rosas C (2015) The effects of thermal acclimation on the behavior, thermal tolerance, and respiratory metabolism in a crab inhabiting a wide range of thermal habitats (Cancer antennarius Stimpson, 1856, the red shore crab). Mar Freshw Behav Physiol 48:89–101. https://doi.org/10.1080/10236244.2015.1019212
Paganini A, Kimmerer W, Stillman J (2010) Metabolic responses to environmental salinity in the invasive clam Corbula amurensis. Aquat Biol 11:139–147. https://doi.org/10.3354/ab00304
Paganini AW, Miller NA, Stillman JH (2014) Temperature and acidification variability reduce physiological performance in the intertidal zone porcelain crab Petrolisthes cinctipes. J Exp Biol 217:3974. https://doi.org/10.1242/jeb.109801
Peck LS, Webb KE, Miller A, Clark MS, Hill T (2008) Temperature limits to activity, feeding and metabolism in the Antarctic starfish Odontaster validus. Mar Ecol Prog Ser 358:181–189
Picard DJ, Schulte PM (2004) Variation in gene expression in response to stress in two populations of Fundulus heteroclitus. Comp Biochem Physiol Mol Integr Physiol 137:205–216
R Team Core (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Re AD, Díaz F, Salas-Garza A, Gonzalez M, Cordero V, Galindo-Sanchez CE, Sanchez-Castrejon E, Zamora AS, Licea-Navarro A (2013) Thermal preference, tolerance and temperature-dependent respiration in the California sea hare. Agric Sci 04:46–52. https://doi.org/10.4236/as.2013.46A007
Russell BD, Connell SD, Findlay HS, Tait K, Widdicombe S, Mieszkowska N (2013) Ocean acidification and rising temperatures may increase biofilm primary productivity but decrease grazer consumption. Philos Trans R Soc B 368:20120438. https://doi.org/10.1098/rstb.2012.0438
Scemes E, Cassola AC (1995) Regulatory volume decrease in neurons of Aplysia brasiliana. J Exp Zool 272:329–337
Sokolova IM (2013) Energy-limited tolerance to stress as a conceptual framework to integrate the effects of multiple stressors. Integr Comp Biol 53:597–608. https://doi.org/10.1093/icb/ict028
Sokolova I, Pörtner H (2001) Physiological adaptations to high intertidal life involve improved water conservation abilities and metabolic rate depression in Littorina saxatilis. Mar Ecol Prog Ser 224:171–186
Sokolova IM, Pörtner H-O (2003) Metabolic plasticity and critical temperatures for aerobic scope in a eurythermal marine invertebrate (Littorina saxatilis, Gastropoda: Littorinidae) from different latitudes. J Exp Biol 206:195–207. https://doi.org/10.1242/jeb.00054
Somero G (2015) Temporal patterning of thermal acclimation: from behavior to membrane biophysics. J Exp Biol 218:167–169
Somero GN, Lockwood BL, Tomanek L (2017) Biochemical adaptation: response to environmental challenges, from life’s origins to the Anthropocene. Sinauer Associates, Incorporated Publishers, Sunderland
Souza MM, Scemes E (2000) Volume changes in cardiac ventricles from Aplysia brasiliana upon exposure to hyposmotic shock. Comp Biochem Physiol A Mol Integr Physiol 127:99–111
Stickle WB, Sabourin TD (1979) Effects of salinity on the respiration and heart rate of the common mussel, Mytilus edulis L., and the black chiton, Katherina tunicata (Wood). J Exp Mar Biol Ecol 41:257–268. https://doi.org/10.1016/0022-0981(79)90135-7
Stillman JH (2019) Heat waves, the new normal: summertime temperature extremes will impact animals, ecosystems, and human communities. Physiology 34:86–100
Tanner R (2018) Predicting Phyllaplysia taylori (Anaspidea: Aplysiidae) presence in Northeastern Pacific estuaries to facilitate grazer community inclusion in eelgrass restoration. Estuar Coast Shelf Sci 214:110–119
Timmermann A, Oberhuber J, Bacher A, Esch M, Latif M, Roeckner E (1999) Increased El Niño frequency in a climate model forced by future greenhouse warming. Nature 398:694
Verhoeven MP, Kelaher BP, Bishop MJ, Ralph PJ (2012) Epiphyte grazing enhances productivity of remnant seagrass patches. Austral Ecol 37:885–892
Walters RA, Cheng RT, Conomos TJ (1985) Time scales of circulation and mixing processes of San Francisco Bay waters. In: Cloern JE, Nichols FH (eds) Temporal dynamics of an estuary: San Francisco Bay. Developments in Hydrobiology, vol 30. Springer, Dordrecht
Walther G-R (2010) Community and ecosystem responses to recent climate change. Philos Trans R Soc B Biol Sci 365:2019–2024
Zimmerman RC (2017) Systems biology and the seagrass paradox: adaptation, acclimation, and survival of marine angiosperms in a changing ocean climate. In: Kumar M, Ralph P (eds) Systems biology of marine ecosystems. Springer, Cham
Zimmerman RC, Hill VJ, Gallegos CL (2015) Predicting effects of ocean warming, acidification, and water quality on Chesapeake region eelgrass. Limnol Oceanogr 60:1781–1804
Acknowledgements
We would like to thank anonymous reviewers for their feedback on the manuscript, Adam Paganini for his aquarium design assistance, Emily Lam for her aquarium maintenance assistance, and Dr. Katharyn Boyer for her advice and expertise on eelgrass ecology in the San Francisco Bay.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests. All animal care and sampling protocols outlined in CA SCP-13357 were followed; no formal national or international guidelines are explicitly stated for the taxon in this study. This work was supported by the San Francisco State University Romberg Tiburon Center Bay Discovery Grant (2015 and 2016 cycles) and the CSU Council on Ocean Affairs, Science and Technology travel Grant (2016 and 2017 cycles) to LEF.
Data accessibility
Data have been uploaded to the PANGAEA Biosphere Database (https://doi.org/10.1594/PANGAEA.903705).
Additional information
Responsible Editor: A. E. Todgham.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by Undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tanner, R.L., Faye, L.E. & Stillman, J.H. Temperature and salinity sensitivity of respiration, grazing, and defecation rates in the estuarine eelgrass sea hare, Phyllaplysia taylori. Mar Biol 166, 109 (2019). https://doi.org/10.1007/s00227-019-3559-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-019-3559-4