Abstract
Separation of carbon dioxide was studied in microporous fiber (HF) membranes using the ethanol solution of PAMAM dendrimers. PAMAM dendrimers (Generations 0–4) are embedded in three membrane contactors called polyvinylidene fluoride (PVDF), polyvinyl chloride (PVC) and polypropylene (PP) separately and responsive. With higher flow rates in the dendrimer fluid, a faster chemical reaction occurs that can accelerate the transfer of carbon dioxide to the alcoholic solution. Flow rate and efficiency increase with increasing fluid flow rates. The emission rate was controlled by the distribution in the liquid section at moderate flow rates. The maximum acquisition flow is achieved at a flow rate in liquids and gases of approximately 0.5 mL / s. Empty fiber membranes can replace columns full of carbon dioxide removal from industrial packing power applications, greater connections between the shell side and the tube side with greater driving force, ease of sealing and easy retrieval, less expensive and smaller space requirement compared to other separation techniques.
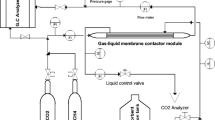
Graphical abstract













Similar content being viewed by others
Abbreviations
- Hollow fiber mass transfer coefficient:
-
\(\begin{aligned}&Sh_L=K_L^\ast\frac{d_e}{D_L}\\&1.62\left(\frac{d_1^2}{LD}v_{absorbent\;tube}\right)^{0.33}\end{aligned}\), m/s
- Fiber diameter:
-
d1, m
- Diffusion coefficient of liquid:
-
DL, m2/s
- Inner fiber diameter:
-
de, m
- Width of the membrane module:
-
D, m
- Fiber length:
-
L, m
- Velocity of the suction tube:
-
Vabsorbent tube, m/s
- Mass transfer coefficient:
-
\(\begin{aligned}&K_L=\beta\ K_L^\ast \\& \left(\beta=\frac{J_{chem}}{J_{phy}}\right)\end{aligned}\), m/s
- Mass transfer constant of chemical absorption:
-
Jchem
- Mass transfer constant of physical diffusion:
-
Jphy
- Transfer coefficient of membrane:
-
\(\begin{aligned}&\mathrm{K}_\mathrm{m}=\mathrm{D}_\mathrm{gas-membrane} \\& \mathrm{Or} \\& \;\frac{\mathrm{D}_\mathrm{gas-shell}}{\delta \times \tau}\times{\varepsilon}\end{aligned}\), m/s
- Membrane mass transfer coefficient:
-
Dgas-membrane, m/s
- Effective diffusion coefficient inside the shell:
-
Dgas-shell, m2/s
- Porosity of the membrane:
-
ε,
- Thickness of the membrane:
-
ẟ,
- Tortuosity of the membrane:
-
τ,
- Gas mass transfer coefficient:
-
\(\begin{aligned}\mathrm{Sh}_\mathrm{g}={\mathrm{k}_\mathrm{g}}\frac{\mathrm{d}_\mathrm{e}}{\mathrm{D}_{\mathrm{gas-shell}}}=5.85(1-\varphi)\frac{\mathrm{d}_\mathrm{e}}{\mathrm{L}}\mathrm{Re}_\mathrm{g}^{0.6}\mathrm{Sc}_\mathrm{g}^{0.33}\end{aligned}\), m/s
- Outer diameter of the fiber:
-
de, m
- Packing density:
-
φ
- Length of the membrane:
-
L, m
- Sherwood number:
-
Sh,
- Reynolds number:
-
Re,
- Schmidt number:
-
Sc,
- Absorbent:
-
i,
- Reaction rate:
-
Ri,
- Diffusion coefficient:
-
Di –tube, m2/s
- Concentration of the species i:
-
Ci- tube, mol/l
- Diffusion coefficient in the membrane:
-
Dgas-membrane, m2/s
- Axial velocity:
-
Vz- shell, m/s
- Gas velocity inside the shell side:
-
Vshell, m/s
- Inner module radius:
-
r, m
- Outer fiber radius:
-
r2, m
- Inner shell radius:
-
r3, m
- Stripping efficiency of the hollow fiber membrane contractor:
-
η,
- Gas concentration of liquid phase at the outlet of the membrane module:
-
CO,l, mmol/L
- Gas concentration of liquid phase at the inlet of the membrane module:
-
Ci,l, mmol/L
- Flux of gas stripping:
-
J, Mol/m2s
- Flow volume of liquid:
-
Ql, m3/s
- Surface area of the hollow fiber membranes:
-
Ai, m2
References
Hussain A, Hägg MB (2010) Hollow Fiber Membrane Contactor absorption of CO2 from the flue gas. J of Mem Sci 359:140148
Mulder M (1996) Basic principles of Membrane Technologies, (https://Z-lib.org)
Yang H, Xua Z, Fanb GM, Slimanec R (2008) Progress in carbon dioxide separation and capture: A review. J of Env Sci 20:1427–1436
Hussain A, Hägg MB (2010) A feasibility study of CO2 capture from flue gas by a facilitated transport Membrane. J of Mem Sci 359:140148
Brunetti A, Scura F, Barbieri G, Drioli E (2010) Membrane technologies for CO2 separation. J of Mem Sci 359:115125
Peters L, Hussain A, Follmann M, Melin T, Hägg MB (2011) CO2 removal from natural gas by employing amine absorption and membrane technology A technical and economic analysis. Chem Eng J 172:952960
Baker RW, Lokhandwal K (2008) Natural gas processing with membranes. Ind and Eng Chem Res 47:2109–2121
Deng L, Hägg MB (2010) Technoeconomic evaluation of biogas upgrading process using CO2 facilitated transport membrane. Int J of Greenhouse Gas Control 4:638–646
Makaruk A, Miltner M, Harasek M (2010) Membrane biogas upgrading processes for the production of natural gas substitute. Sep and Pur Technol 74:8392
Pakizeh M, Mansoori SA, Pourafshari A, Chenar M, Namvar MM (2013) Modification of PSf membrane nanostructure using different fabrication parameters and investigation of the CO2 separation properties of PDMS coated PSf composite membranes. Brazilian J of Chem Eng 30(2):345357
Ramadan KM, Qisieh O, Tlili I (2022) Thermal creep effects on fluid flow and heat transfer in a microchannel gas cooling. Part C: J Mech Eng Sci. https://doi.org/10.1177/09544062211057039
Nayak MK, Fazle Mabood AS, Dogonchi KM, Ramadan IT, Khan WA (2022) Entropy optimized assisting and opposing non-linear radiative flow of hybrid nanofluid. Waves Random Complex Media. https://doi.org/10.1080/17455030.2022.2032474
Gao J, Liu J, Yue H, Zhao Y, Tlili I, Karimipour A (2022) Effects of various temperature and pressure initial conditions to predict the thermal conductivity and phase alteration duration of water based carbon hybrid nanofluids via MD approach. J Mol Liq. https://doi.org/10.1016/j.molliq.2022.118654
Iskander Tlili Md, Sajadi DB, Ghaemi F (2022) Flat sheet direct contact membrane distillation study to decrease the energy demand for solar desalination purposes. Sustainable Energy Technol Assess. https://doi.org/10.1016/j.seta.2022.102100
Zhang Y, Wang Z, Wang SC (2002) Selective permeation of CO2 through new facilitated transport membranes. Desalination 145:385–388
Xuezhong H, Linfeng L, Yunhan C (2018) Facilitated transport membranes for CO2 removal from natural gas In: Angelo B and Evangelos P F (eds.), Current Trends and Future Developments on (Bio-) Membranes Carbon Dioxide Separation/Capture by Using Membranes, Elsevier, https://doi.org/10.1016/B978-0-12-813645-4.01001-1, pp 261-288
Zhang ZE, Yan YF, Zhang L, Ju XA (2014) Hollow Fiber Membrane Contactor absorption of CO2 from the flue gas. Global NEST J 16:355–374
Sisakht MR, Rana D, Matsuura T, Emadzadeh D, Padaki M, Ismail AF (2014) Study on CO2 stripping from water through novel surface modified PVDF hollow fiber membrane contactor. Chem Eng J 246:306–310
Zabih A, Tarsa S, Ali Asghar H, Rahbari-Sisakht M (2015) Fabrication and Characterization of Polyetherimide Hollow Fiber Membrane Contactor for Carbon Dioxide Stripping from Monoethanolamine Solution. J Mem Sci Res 1:118–123
Acknowledgements
Authors acknowledged DST New Delhi for financial grant vide grant no. SR/S3/CE/001/2013 and Director CSIR-NEIST, Jorhat for his keen interest on the subject.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bharali, P., Das, I., Sarmah, H. et al. Stripping of carbon dioxide from ethanol solution of PAMAM dendrimer using hollow Fibre membrane contactor. Heat Mass Transfer 59, 299–308 (2023). https://doi.org/10.1007/s00231-022-03244-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-022-03244-9