Abstract
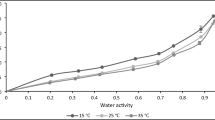
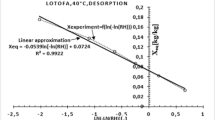
This research aimed to experimentally determine moisture sorption characteristics and mechanical thermal properties of different parts of red water lily (Nymphaea x rubra). The data obtained from dynamic vapor sorption (DVS) were modeled with six sorption isotherm models. The shape of sorption isotherms of dried petiole and rhizome was classified as Type III and II, respectively. Peleg model was the best fit with the experimental data. GAB and BET models were used to estimate monolayer moisture content (M0) of the samples and M0 of petiole ranged between 7.17 to 8.291% d.b. and 10.455 to 10.588% d.b. for GAB and BET models, respectively and M0 of rhizome ranged between 6.208 to 7.741% d.b. and 3.566 to 3.669% d.b. for GAB and BET models, respectively. Blahovec-Yanniotis model was used to describe the amount of bounded water and solution water in material and the contribution of solution water played an important role in both adsorption and desorption processes of dried petiole and rhizome. Dried red water lilies were equilibrated at different relative humidity levels. Dynamic mechanical thermal analysis (DMTA) was used to estimate the glass transition of the samples at different water activities. Increasing the solicitation frequency shifted the temperature of the relaxation to a higher temperature and Arrhenius equation described well the frequency dependency of the transition temperature. The apparent activation energies (Ea) of dried petiole and rhizome were in the range from 217.98 to 248.49 kJ/mol and 187.34 to 230.30 kJ/mol, respectively.







Similar content being viewed by others
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- A, B, C, a, a1, a2, b, b1, b2 c, d:
-
Model constants
- AOAC:
-
Association of Official Analytical Chemists
- aw :
-
Water activity
- \({B}_{x}\) :
-
Bias limit in a predicted parameter
- d.b.:
-
Dry basis
- \(df\) :
-
Degree of freedom
- DSC:
-
Differential scanning calorimetry
- DVS:
-
Dynamic vapor sorption
- DMTA:
-
Dynamic mechanical thermal analysis
- Ea :
-
Apparent activation energy (kJ/mol)
- E′:
-
Storage modulus (Pa)
- E″:
-
Loss modulus (Pa)
- \(f\) :
-
Frequency (Hz)
- \({k}_{0}\) :
-
Rate of moisture changing for zero order equation (% d.b. /min)
- \({k}_{1}\) :
-
Rate of moisture changing for first order equation (% d.b. /min)
- \({k}_{2}\) :
-
Kinetic parameters of moisture sorption process
- \({k}_{3}\) :
-
Kinetic parameters of diffusion process
- Me :
-
Equilibrium moisture content (% d.b.)
- Mo :
-
Monolayer moisture content (% d.b.)
- \(m\) :
-
Moisture content at any time (% d.b.)
- \({m}_{0}\) :
-
Moisture content of sample at time zero (% d.b.)
- \(N\) :
-
Number of data
- \(n\) :
-
Number of model parameter
- \({P}_{x}\) :
-
Precision limit for a sample
- R:
-
Universal gas constant (8.314 J/mol K)
- \({R}^{2}\) :
-
Co-efficient of determination
- RH:
-
Relative humidity (%)
- RHe :
-
Equilibrium relative humidity (%)
- RMSE:
-
Root mean square error
- SEE:
-
Standard error of estimate
- \({S}_{x}\) :
-
Precision index
- T:
-
Temperature of tan δ maximum (K)
- tan δ:
-
E″/ E′
- Tg :
-
Glass transition temperature (ºC)
- t :
-
Process time (min)
- w.b.:
-
Wet basis
- \({w}_{x}\) :
-
Overall uncertainty in a predicted parameter
- \(\overline{x }\) :
-
Mean of sample population
- x 1 , x 2 , … , x n :
-
Independent parameters
- χ2 :
-
Chi-square
- \(Y\) :
-
Experimental data
- \(\overline Y\) :
-
Mean of sample population
- \(Y'\) :
-
Predicted data
References
Lim TK (2006) Edible medicinal and non-medicinal plants: volume 11, Modified stems, roots and bulbs. Springer International Publishing, Switzerland
Devi SA, Thongam B, Handique PJ (2015) Nymphaea rubra Roxb. ex Andrews cultivated as an ornamental, food and vegetable in the North Eastern region of India. Genet Resour Crop Evol 62:315–320. https://doi.org/10.1007/s10722-014-0177-3
Barbosa-Canovas GV, Fontana AJ, Schmidt SJ, Labuza TP (2007) Water Activity in Foods: Fundamentals and Applications. John Wiley & Sons, New Jersey. https://doi.org/10.1002/9780470376454
Cladera-Olivera F, Pettermann AC, Zapata-Norena CP, Wada K, Ferreira-Marczakm LD (2008) Thermodynamic properties of moisture desorption of raw pinhao (Araucaria angustifolia seeds). Int J Food Sci Technol 43:900–907. https://doi.org/10.1111/j.1365-2621.2007.01540.x
Brunauer S, Deming WE, Troller E (1940) On the theory of Van der Waals adsorption of gases. J Am Chem Soc 62:1723–1732. https://doi.org/10.1021/ja01864a025
Chirife J, Iglesias HA (1978) Equations for fitting water sorption isotherms of foods: Part 1—A review. J Food Technol 13:159–174. https://doi.org/10.1111/j.1365-2621.1978.tb00792.x
Peleg M (1993) Assessment of a semi-empirical four parameter general model for sigmoid moisture sorption isotherms. J Food Process Eng 16:21–37. https://doi.org/10.1111/j.1745-4530.1993.tb00160.x
Pickett G (1945) Modification of the Brunauer-Emmett-Teller theory of multimolecular adsorption. J Am Chem Soc 67:1958–1962. https://doi.org/10.1021/ja01227a027
Van den Berg C, Bruin S (1981) Water activity and its estimation in food systems: theoretical aspects. In: Rockland LB, Stewart GF (eds) Water activity: Influences on food quality. Academic, New York, pp 147–177
Blahovec J, Yanniotis S (2009) Modified classification of sorption isotherms. J Food Eng 91:72–77. https://doi.org/10.1016/j.jfoodeng.2008.08.007
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319. https://doi.org/10.1021/ja01269a023
Mrad ND, Bonazzi C, Boudhrioua N, Kechaou N, Courtois F (2012) Moisture sorption isotherms, thermodynamic properties, and glass transition of pears and apples. Dry Technol 30:1397–1406. https://doi.org/10.1080/07373937.2012.683843
Menard KP (2008) Dynamic mechanical analysis: A practical introduction, 2nd ed. CRC Press, Boca Roton. https://doi.org/10.1201/9781420053135
Greenspan L (1977) Humidity fixed points of binary saturated aqueous solutions. J Res Natl Bur Stand 81A:89–96. https://doi.org/10.6028/jres.081A.011
AOAC International (AOAC) (2000) Association of Official Analytical Chemist, Official methods of analysis of AOAC international, 17th edn. AOAC international, Gaithersburg, MD
Phahom T, Kerr WL, Pegg RB, Phoungchandang S (2017) Effect of packaging types and storage conditions on quality aspects of dried Thunbergia laurifolia leaves and degradation kinetics of bioactive compounds. J Food Sci Technol 54:4405–4415. https://doi.org/10.1007/s13197-017-2917-9
Venegas MJ, Fregoso-Israel E, Escamilla R, Pfeiffer H (2007) Kinetic and reaction mechanism of CO2 sorption on Li4SiO4: Study of the particle size effect. Ind Eng Chem Res 46:2407–2412. https://doi.org/10.1021/ie061259e
Diab T, Biliaderis CG, Gerasopoulos D, Sfakiotakis E (2001) Physicochemical properties and application of pullulan edible films and coatings in fruit preservation. J Sci Food Agric 81:988–1000. https://doi.org/10.1002/jsfa.883
Belghith A, Azzouz S, ElCafsi A (2016) Desorption isotherms and mathematical modeling of thin layer drying kinetics of tomato. Heat Mass Transf 52:407–419. https://doi.org/10.1007/s00231-015-1560-0
Ghnimi T, Hassini L, Bagane M (2016) Experimental study of water desorption isotherms and thin-layer convective drying kinetics of bay laurel leaves. Heat Mass Transf 52:2649–2659. https://doi.org/10.1007/s00231-016-1770-0
Hami AE, Pougnet P (2015) Embedded mechatronic system 2: Analysis of failures, modeling, simulation and optimization. ISTE Press – Elsevier. https://doi.org/10.1016/C2014-0-04711-9
Özahi E, Demir H (2014) Presentation of a test rig with its experimental procedure and uncertainty analysis of measurements for batch type fluidized bed drying of corn and unshelled pistachio nut. Measurement 53:117–127. https://doi.org/10.1016/j.measurement.2014.03.043
Darici S, Kilic A (2020) Comparative study on the performances of solar air collectors with trapezoidal corrugated and flat absorber plates. Heat Mass Transf 56:1833–1843. https://doi.org/10.1007/s00231-020-02815-y
Mbarek R, Mihoubi D (2019) Thermodynamic properties and water desorption isotherms of Golden Delicious apples. Heat Mass Transf 55:1405–1418. https://doi.org/10.1007/s00231-018-2527-8
Cheng X, Adhikari B, Xie A, Jiang H, Xu S, Jai Q (2020) Moisture sorption behaviour and thermodynamic properties of adsorbed water of Jerusalem artichoke (Helianthus tuberosus L.) powder. Int Food Res J 27:505–515
Witczak T, Witczak M, Socha R, Pien AS, Grzesik M (2017) Candied orange peel produced in solution with various sugar compositions: sugar composition and sorption properties of the product. J Food Process Eng 40:1–12. https://doi.org/10.1111/jfpe.12367
Chang LS, Karim R, Mohammed AS, Chai KF, Ghazali HM (2019) Moisture sorption isotherm and shelf-life prediction of anticaking agent incorporated spray-dried soursop (Annona muricata L.) powder. J Food Process Eng 42:1–10. https://doi.org/10.1111/jfpe.13134
Thalerngnawacharta S, Duangmala K (2016) Influence of humectants on the drying kinetics, water mobility, and moisture sorption isotherm of osmosed air-dried papaya. Dry Technol 34:574–583. https://doi.org/10.1080/07373937.2015.1064942
Henriot CP, Cuenot Q, Levrey LH, Loup C, Chiarello L, Masclaux H, Bornette G (2019) Relationships between key functional traits of the waterlily Nuphar lutea and wetland nutrient content. Peer J 7:e7861. https://doi.org/10.7717/peerj.7861
Hawa LC, Ubaidillah U, Damayanti R, Hendrawan Y (2020) Moisture sorption isotherms of modified cassava flour during drying and storage. Heat Mass Transf 56:2389–2396. https://doi.org/10.1007/s00231-020-02866-1
Khawas P, Deka SC (2017) Moisture sorption isotherm of underutilized culinary banana flour and its antioxidant stability during storage. J Food Process Preserv 41:1–10. https://doi.org/10.1111/jfpp.13087
Bo L, Rui L, Haiyan G, Wang S (2017) Moisture sorption characteristics of full fat and defatted pistachio kernel flour. Int J Agric Biol Eng 10:283–294. https://doi.org/10.3965/j.ijabe.20171003.2838
Sun X, Jin X, Fu N, Chen X (2020) Effects of different pretreatment methods on the drying characteristics and quality of potatoes. Food Sci Nutr 8:5767–5775. https://doi.org/10.1002/fsn3.1579
Heldman DR, Lund DB (1992) Handbook of food engineering. Marcel Dekker, New York
Al-Muhtaseb AH, McMinn WAM, Magee TRA (2002) Moisture sorption isotherm characteristics of food products: A review. Trans Inst Chem Eng 80:118–128. https://doi.org/10.1205/09603080252938753
Aguirre-Alvarez G, Foster T, Hill SE (2013) Modelling of isotherms and their hysteresis analysis in gelatin from different sources. CYTA - J Food 11:68–74. https://doi.org/10.1080/19476337.2012.692122
Srikiatden J, Roberts JS (2007) Moisture transfer in solid food materials: A review of mechanisms, models and measurements. Int J Food Prop 10:739–777. https://doi.org/10.1080/10942910601161672
Brett B, Figueroa M, Sandoval AJ, Barreiro JA, Muller AJ (2009) Moisture sorption characteristics of starchy products: oat flour and rice flour. Food Biophys 4:151–157. https://doi.org/10.1007/s11483-009-9112-0
Xing C, Liu X, Jin Q, Li J, Huang J, Liu Y, Wang X (2012) Moisture sorption thermodynamics of Camellia oleifera. Food Biophys 7:163–172. https://doi.org/10.1007/s11483-012-9254-3
Arslan-Tontul S (2021) Moisture sorption isotherm and thermodynamic analysis of quinoa grains. Heat Mass Transf 57:543–550. https://doi.org/10.1007/s00231-020-02978-8
Moussaoui H, Kouhila M, Lamsyehe H, Idlimam A, Lamharrar A (2020) Moisture sorption measurements and thermophysical characterization of the Taraxacum officinale leaves and root. Heat Mass Transf 56:2065–2077. https://doi.org/10.1007/s00231-020-02838-5
Cervenka L, Kubinova J, Juszczak L, Witczak M (2012) Moisture sorption isotherms and glass transition temperature of elecampe (Inula helenium L.) and burdock (Arctium lappa L.) roots at 25°C. Food Sci Technol Int 18:81–91. https://doi.org/10.1177/1082013211414260
Rockland LB (1969) Water activity and storage stability. Food Technol 23:1241–1249
Yanniotis S, Blahovec J (2009) Model analysis of sorption isotherms. LWT - Food Sci Technol 42:1688–1695. https://doi.org/10.1016/j.lwt.2009.05.010
Dalgıç AC, Pekmez H, Belibağlı KB (2012) Effect of drying methods on the moisture sorption isotherms and thermodynamic properties of mint leaves. J Food Sci Technol 49:439–449. https://doi.org/10.1007/s13197-011-0302-7
Zeymer JS, Correa PC, Oliveira GHH, Baptestini FM, Campos RC (2019) Mathematical modeling and hysteresis of sorption isotherms for paddy rice grains. Engenharia Agricola 39:524–532. https://doi.org/10.1590/1809-4430-Eng.Agric.v39n4p524-532/2019
Kapsalis JG (1987) Influences of hysteresis and temperature on moisture sorption isotherms. In: Rockland LB, Beuchat LR (eds) Water activity: Theory and applications to food. Marcel Dekker Inc, New York, pp 173–213
Johnson PNT, Brennan JG (2000) Kinetics of moisture absorption by plantain flour. J Food Eng 45:33–36. https://doi.org/10.1016/S0260-8774(00)00038-8
Doymaz I (2014) Mathematical modeling of drying of tomato slices using infrared radiation. J Food Process Preserv 38:389–396. https://doi.org/10.1111/j.1745-4549.2012.00786.x
Phahom T, Phoungchandang S (2018) Drying characteristics and quality attributes of Thunbergia laurifolia leaves using microwave drying. APST 23:1–12. https://doi.org/10.14456/apst.2018.24
Phahom T, Juntharat N, Premsuttarat P, Paosunthia Y, Roudaut G (2021) Evaluation of desorption isotherms, drying characteristics and rehydration properties of crab stick by-product. Heat Mass Transf. https://doi.org/10.1007/s00231-020-02982-y
Roudaut G, Maglione M, Dusschoten DV, Meste ML (1999) Molecular mobility in glassy bread: A multispectroscopy approach. Cereal Chem 76:70–77. https://doi.org/10.1094/CCHEM.1999.76.1.70
Bai Y, Jin L (2008) Characterization of frequency-dependent glass transition temperature by Vogel-Fulcher relationship. J Phys D Appl Phys 41:1–4. https://doi.org/10.1088/0022-3727/41/15/152008
Yin P, Dong X, Zhou W, Zha D, Xu J, Guo B, Li P (2020) A novel method to produce sustainable biocomposites based on thermoplastic corn-starch reinforced by polyvinyl alcohol fibers. RSC Adv 10:23632–23643. https://doi.org/10.1039/D0RA04523C
Acknowledgements
The authors would like to thank the junior research fellowships program of The French Embassy in Bangkok for the financial support, in cooperation between L’Institut Agro Dijon and Suranaree University of Technology.
Funding
This research was supported by the junior research fellowships program of The French Embassy in Bangkok in cooperation between L’Institut Agro Dijon and Suranaree University of Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interests
On behalf of all authors, the corresponding author declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Phahom, T., Roudaut, G. Moisture sorption characteristics and dynamic mechanical thermal analysis of dried petiole and rhizome of red water lily (Nymphaea x rubra). Heat Mass Transfer 59, 309–328 (2023). https://doi.org/10.1007/s00231-022-03258-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-022-03258-3