Abstract
Introduction
The goal of this study was to assess the changes in arterial spin labeling (ASL) cerebral blood flow (CBF) and arterial transit time (ATT), and in apparent diffusion coefficient (ADC), before and after an acetazolamide challenge in moyamoya patients, as function of arterial stenosis severity.
Methods
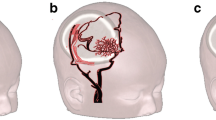
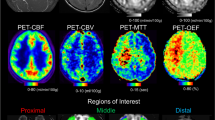
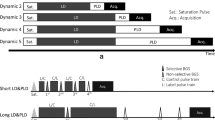
Pre-operative patients diagnosed with moyamoya disease who could undergo MRI at 3.0T were recruited for this study. A multi-delay pseudo-continuous ASL and a diffusion-weighted sequence were acquired before and 15 min after acetazolamide injection. The severity of anterior, middle, and posterior cerebral artery pathology was graded on time-of-flight MR angiographic images. CBF, ATT, and ADC were measured on standardized regions of interest as function of the vessel stenosis severity.
Results
Thirty patients were included. Fifty-four percent of all vessels were normal, 28% mildly/moderately stenosed, and 18% severely stenosed/occluded. Post-acetazolamide, a significantly larger CBF (ml/100 g/min) increase was observed in territories of normal (+19.6 ± 14.9) compared to mildly/moderately stenosed (+14.2 ± 27.2, p = 0.007), and severely stenosed/occluded arteries (+9.9 ± 24.2, p < 0.0001). ATT was longer in territories of vessel anomalies compared with normal regions at baseline. ATT decreases were observed in all territories post-acetazolamide. ADC did not decrease after acetazolamide in any regions, and no correlation was found between ADC changes and baseline ATT, change in ATT, or CVR.
Conclusion
The hemodynamic response in moyamoya disease, as measured with ASL CBF, is impaired mostly in territories with severe arterial stenosis/occlusion, while ATT was prolonged in all non-normal regions. No significant changes in ADC were observed after acetazolamide.




Similar content being viewed by others
References
Takeuchi K, Shimizu K (1957) Hypoplasia of the bilateral internal carotid arteries. Brain Nerve 9:37–43
Vagal AS, Leach JL, Fernandez-Ulloa M, Zuccarello M (2009) The acetazolamide challenge: techniques and applications in the evaluation of chronic cerebral ischemia. AJNR Am J Neuroradiol 30:876–884
Noguchi T, Kawashima M, Nishihara M, Egashira Y, Azama S, Irie H (2015) Noninvasive method for mapping CVR in moyamoya disease using ASL-MRI. Eur J Radiol 84:1137–1143
Gupta A, Chazen JL, Hartman M et al (2012) Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: a systematic review and meta-analysis. Stroke 43:2884–2891
Antonucci MU, Burns TC, Pulling TM et al (2015) Acute preoperative infarcts and poor cerebrovascular reserve are independent risk factors for severe ischemic complications following direct extracranial-intracranial bypass for moyamoya disease. AJNR Am J Neuroradiol. doi:10.3174/ajnr.A4535
Kim TJ, Lee JS, Hong JM, Lim YC (2012) Intracerebral steal phenomenon: a potential mechanism for contralateral stroke after carotid artery stenting. Neurologist 18:128–129
McDonald RJ, McDonald JS, Kallmes DF et al (2015) Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275:772–782
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270:834–841
Alsop DC, Detre JA, Golay X et al (2015) Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73:102–116
Dai W, Robson PM, Shankaranarayanan A, Alsop DC (2012) Reduced resolution transit delay prescan for quantitative continuous arterial spin labeling perfusion imaging. Magn Reson Med 67:1252–1265
Wang R, Yu S, Alger JR et al (2014) Multi-delay arterial spin labeling perfusion MRI in moyamoya disease—comparison with CT perfusion imaging. Eur Radiol 24:1135–1144
Uchino H, Ito M, Fujima N et al (2015) A novel application of four-dimensional magnetic resonance angiography using an arterial spin labeling technique for noninvasive diagnosis of moyamoya disease. Clin Neurol Neurosurg 137:105–111
http://www.bic.mni.mcgill.ca/ServicesSoftware/MINC. Accessed 04/15/2015
Kim JJ, Fischbein NJ, Lu Y, Pham D, Dillon WP (2004) Regional angiographic grading system for collateral flow: correlation with cerebral infarction in patients with middle cerebral artery occlusion. Stroke 35:1340–1344
Fonov V, Evans AC, Botteron K et al (2011) Unbiased average age-appropriate atlases for pediatric studies. NeuroImage 54:313–327
Piepgras A, Schmiedek P, Leinsinger G, Haberl RL, Kirsch CM, Einhaupl KM (1990) A simple test to assess cerebrovascular reserve capacity using transcranial Doppler sonography and acetazolamide. Stroke 21:1306–1311
Federau C, O'Brien K, Meuli R, Hagmann P, Maeder P (2014) Measuring brain perfusion with intravoxel incoherent motion (IVIM): initial clinical experience. J Magn Reson Imaging 39:624–632
Errante Y, Cirimele V, Mallio CA, Di Lazzaro V, Zobel BB, Quattrocchi CC (2014) Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Investig Radiol 49:685–690
Robert P, Lehericy S, Grand S et al (2015) T1-weighted hypersignal in the deep cerebellar nuclei after repeated administrations of gadolinium-based contrast agents in healthy rats: difference between linear and macrocyclic agents. Investig Radiol 50:473–480
Yun TJ, Paeng JC, Sohn CH et al (2015) Monitoring cerebrovascular reactivity through the use of arterial spin labeling in patients with moyamoya disease. Radiology. doi:10.1148/radiol.2015141865:141865
Noguchi T, Kawashima M, Nishihara M, Hirai T, Matsushima T, Irie H (2013) Arterial spin-labeling MR imaging in moyamoya disease compared with clinical assessments and other MR imaging findings. Eur J Radiol 82:e840–e847
Sugino T, Mikami T, Miyata K, Suzuki K, Houkin K, Mikuni N (2013) Arterial spin-labeling magnetic resonance imaging after revascularization of moyamoya disease. J Stroke Cerebrovasc Dis 22:811–816
Zaharchuk G, Do HM, Marks MP, Rosenberg J, Moseley ME, Steinberg GK (2011) Arterial spin-labeling MRI can identify the presence and intensity of collateral perfusion in patients with moyamoya disease. Stroke 42:2485–2491
Wintermark M, Sesay M, Barbier E et al (2005) Comparative overview of brain perfusion imaging techniques. Stroke 36:e83–e99
Heijtel DF, Petersen ET, Mutsaerts HJ et al (2016) Quantitative agreement between [(15) O]H2 O PET and model free QUASAR MRI-derived cerebral blood flow and arterial blood volume. NMR Biomed 29:519–526
Tsujikawa T, Kimura H, Matsuda T et al (2016) Arterial transit time mapping obtained by pulsed continuous 3D ASL imaging with multiple post-label delay acquisitions: comparative study with PET-CBF in patients with chronic occlusive cerebrovascular disease. PLoS One 11:e0156005
Acknowledgements
CF was supported by the Swiss National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We declare that all human and animal studies have been approved by the Stanford University Ethics Committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest
SC consults for iSchemaView and GZ receives research support from GE Healthcare.
Rights and permissions
About this article
Cite this article
Federau, C., Christensen, S., Zun, Z. et al. Cerebral blood flow, transit time, and apparent diffusion coefficient in moyamoya disease before and after acetazolamide. Neuroradiology 59, 5–12 (2017). https://doi.org/10.1007/s00234-016-1766-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-016-1766-y