Abstract
This study compared the effectiveness of four arbuscular mycorrhizal (AM) fungal isolates (two autochthonous presumably drought-tolerant Glomus sp and two allochthonous presumably drought-sensitive strains) on a drought-adapted plant (Lavandula spica) growing under drought conditions. The autochthonous AM fungal strains produced a higher lavender biomass, specially root biomass, and a more efficient N and K absorption than with the inoculation of similar allochthonous strains under drought conditions. The autochthonous strains of Glomus intraradices and Glomus mosseae increased root growth by 35% and 100%, respectively, when compared to similar allochthonous strains. These effects were concomitant with an increase in water content and a decline in antioxidant compounds: 25% glutathione, 7% ascorbate and 15% H2O2 by G. intraradices, and 108% glutathione, 26% ascorbate and 43% H2O2 by G. mosseae. Glutathione and ascorbate have an important role in plant protection and metabolic function under water deficit; the low cell accumulation of these compounds in plants colonized by autochthonous AM fungal strains is an indication of high drought tolerance. Non-significant differences between antioxidant activities such as glutathione reductase (GR), catalase (CAT) and superoxide dismutase (SOD) in colonized plants were found. Thus, these results do not allow the generalization that GR, CAT and SOD were correlated with the symbiotic efficiency of these AM fungi on lavender drought tolerance. Plants colonized by allochthonous G. mosseae (the less efficient strain under drought conditions) had less N and K content than those colonized by similar autochthonous strain. These ions play a key role in osmoregulation. The AM symbiosis by autochthonous adapted strains also produced the highest intraradical and arbuscular development and extraradical mycelial having the greatest fungal SDH and ALP-ase activities in the root systems. Inoculation of autochthonous drought tolerant fungal strains is an important strategy that assured the greatest tolerance water stress contributing to the best lavender growth under drought.
Similar content being viewed by others
Introduction
The decline of plant growth mainly caused by water deficiency is considered to be one of the most important ecological factors limiting plant survival and establishment. Stress affects most plants growing in arid and semiarid regions. Nevertheless, autochthonous microbial communities are able to develop a range of activities that may contribute in improving plant growth [7]. Plant roots and soil form an interface where symbiotic microorganisms such as arbuscular–mycorrhizal (AM) fungi interact. In drought-stressed areas, plants are highly dependent on microbial activity because beneficial microorganisms (e.g., AM fungi) are able to enhance their metabolic activity to tolerate this environmental stress [4, 33]. Plants possess natural protection systems against stresses but their interaction with soil microorganisms can alleviate symptoms, which are important in drought-stress protection [22, 47]. The role of AM symbiosis in growth, drought-tolerance and plant establishment under nutritional and water-stress conditions is based on a range of physiological and cellular mechanisms [33]. There is evidence that AM fungi help plants grow under arid conditions by reducing water stress [39] and by increasing the supply of nutrients.
Natural climatic—or anthropically created—conditions cause or accelerate desertification, which drastically affects growth and survival of plants [19]. Lavender plants (Lavandula spp.) are small woody shrubs adapted communities of Mediterranean ecosystems [6]. Nevertheless, successful reestablishment of native plants in degraded soils are normally limited, partly because of the low density of AM propagules, an important biological factor governing both nutrients cycles and soil fertility in arid and semiarid ecosystems [8]. Because of the low potential of native inoculum of AM fungi in the semiarid soils in southeast Spain [16, 34], the selection of the most efficient and adapted AM fungi is an important concern in inoculation programs. There is a range in the levels of compatibility and fungal abilities between AM fungi and host plant [29].
The inoculation of lavender plants with drought-resistant AM fungi before planting on drought-affected sites favors plant growth and survival [5]. A living organism is considered resistant if it tolerates a given physicochemical stress. AM fungi help to improve host tolerance to water limitation, but little information is available on the role of AM fungal ecotypes from drought-affected environments. Marulanda et al. [27] and Ruíz-Lozano et al. [28, 40] suggested that Glomus intraradices was a potential candidate species to use under conditions of water stress. Nevertheless, the AM species previously tested as the most effective in dry soils were selected from non-stressed sites. In our group, previous investigation on osmotic stress tolerance of AM colonized plants have shown inter- and intraspecific growth differences between AM fungal isolates from non-water stressed and stressed sites [27, 38, 40]. Different isolates of AM fungi showed high variability in their tolerance to drought and the fungal isolates within one species may vary in their symbiotic effectiveness depending on the compatibility among the fungus, the soil conditions, and the plant in the rhizosphere niche. Autochthonous fungal isolates from dry soils have potentially undergone selection for adaptation to water limitation and other stresses. It was hypothesized that these isolates are more resistant to drought than the species isolated from a non-stressed site.
Regarding the particular role of AM fungi in increasing plant drought tolerance, a logical step is the selection of the most effective AM fungus for plants growing under such conditions. Water deficit affects plant metabolic activities, and one of its earliest consequences is the production of reactive oxygen species that have deleterious effects on cell integrity and functioning [37]. This constraint includes biochemical adaptations like the modulation of antioxidant enzyme activities in cells. These antioxidant enzymes activities play crucial roles in plant protection against stresses. Plant cells contain an array of protective systems that reduce oxidative damage. Some of these systems, such as superoxide dismutase, ascorbate peroxidase and catalase, react with active forms of oxygen and are able to keep them at low levels. This group of enzymes is involved in the detoxification of superoxide radical and H2O2 and thus are able to prevent the formation of OH− radicals. However, glutathione reductase (GR) and ascorbate are able to regenerate antioxidants. These enzymes are components of the ascorbate glutathione pathway responsible for the removal of H2O2 in cellular compartments.
Based on the fact that natural revegetation is scarce in arid and semiarid environments because water scarcity limits plant growth, lavender, a low-growing shrub well adapted to water stress conditions, was used in this study. This species belongs to the natural succession in certain plant communities of semiarid Mediterranean ecosystems.
In the present research, we study the symbiotic effectiveness of four mycorrhizal strains (two autochthonous [isolated from dry soils] and two allochthonous [isolated from a non-dry soils] strains of Glomus mosseae and G. intraradices) in Lavandula spica subjected to water deficit. The aim was to select the most effective AM association, under water stress conditions, to enhance the establishment and growth of lavender for revegetation in water-limited areas. In addition, we investigated the physiological and biochemical basis of effective and tolerant symbiosis to assess the mechanisms involved in the variability of the symbiotic performance.
Materials and Methods
Experimental Design
The experimental design consisted of a complete randomized factorial block with the mycorrhizal inoculations: two autochthonous fungal strains (G. intraradices or G. mosseae) from Mediterranean soils and two allochthonous G. intraradices (BEG 123) or G. mosseae (BEG 119) from EEZ collection. All these treatments were replicated five times with a total of 20 pots.
Soil Characteristics
A calcareous loamy soil was collected from a Mediterranean zone (Granada province, Spain) was sieved (2 mm), diluted with quartz-sand (<1 mm) (5:2, soil/sand v/v), and sterilized by steaming (100°C for 1 h for 3 days). The soil had a pH of 7.2 (water); 1.6% organic matter, nutrient concentrations (μg kg−1) N, 2.1 (Kjeldahl, [11]); P, 1.7 (NaHCO3 extractable P) and K, 0.8 (Olsen, [42]). The soil texture was made up of 57.8% sand, 19% clay and 23.2% silt.
Biological Materials
Lavandula spica seeds were surface-sterilized in a 15% H2O2 for 8 min, then washed several times with sterile water to remove any trace of chemical that could interfere in seed germination and placed on sterile vermiculite at 25°C to germinate. Seedlings were transferred to plastic pots containing 500 g of the sterilized soil/sand mixture.
Two predominant autochthonous fungi were isolated from natural soil by wet sieving and decanting [46]. They were morphologically identified as G. intraradices or G. mosseae strains and were cultivated for inocula production following the conventional procedure [28]. Strains of G. intraradices (BEG 123) or G. mosseae (BEG 119) from our collection (EEZ) were used as allochthonous reference strains. These Glomus species were isolated from a soil located in a non-dry area. Mycorrhizal inocula used were similarly obtained for each one of the AM fungus applied as inoculum. Mycorrhizal inoculum were bulked in an open pot culture of Zea mays plus red clover and consisted of soil, spores, mycelia and colonized root fragments having an AM colonization of about 75%. Ten grams of inoculum were added to appropriate pots at transplanting just below the lavender seedlings.
Growth Conditions
The lavender plants were grown for 6 months in a greenhouse under controlled climatic conditions (18–24°C, with an 18 h/6 h light/dark period and 50% of relative humidity). A photoperiod of 16 h at a photosynthetic photon flux density (PPFD) of 350 μmol m−2 s−1 as measured with a light meter (model LI-188B; Licor Inc., Lincoln, NE, USA) was maintained throughout the experiment. Water was supplied daily to maintain constant soil water content close to water-holding capacity during the first 6 weeks of plant growth. At this time plants were allowed to dry until soil water content was 75% of water-holding capacity and maintained under these conditions for additional 20 weeks. To achieve that, the soil moisture in the pots was measured every 24 h and water was added to reach a maximum of 75% of the water-holding capacity. However, during the 24-h period the soil water content between each rewatering was progressively decreased until a minimum value of 70% of water-holding capacity was reached. Soil moisture was measured with an ML2× ThetaProbe (AT Delta-T Devices, Ltd., Cambridge, UK), which measures volumetric soil moisture content by responding to changes in the apparent dielectric constant of moist soil [2, 36, 48]. This volumetric soil moisture is considered to be a normal environmental condition in dry Mediterranean areas. At harvest, shoot and root dry biomass were measured. The aerial and root parts were dried 48 h in a forced oven at 70°C.
Measurements
Biomass and Nutrients
The plants were harvested 6 months after transplanting. Dry biomass of shoot and the root, the root length, the glomalin and symbiotic development were determined (see below). Shoot concentrations of N (micro-Kjeldahl) and K [24] were measured.
Shoot and root water content were also determined and calculated following the formula:
Mycorrhizal Colonization
The roots were carefully washed and then divided into three batches: one was stained by trypan blue (TB) after cleaning washed roots in 10% KOH and staining with 0.05% trypan blue in lactophenol (v/v) according to Phillips and Hayman [32] and used for root length measurements with the Delta-T Image Analysis System (Cambridge, England). The roots were scanned and analyzed using DIAS Root length software; the remaining roots were used for histochemical vital staining using succinate dehydrogenase (SDH) or alkaline phosphatase (ALP) to measure total (TB), living (SDH) or active (ALP) AM fungal development.
SDH and ALP activities were determined according to the procedure described by Smith et al. [41], Tisserant et al. [41, 44]. Root fragments were then stained overnight at room temperature and cleared for 15–20 min in 1% active chlorine solution in sodium hypochlorite.
Mycorrhizal development, was also evaluated [45] and expressed as intensity of AM colonization M; which gives an estimation of the amount of root cortex that became mycorrhiza and is referred to the whole root system. The value of M% expresses the cortical cells occupied by AM fungal structures in 100 cm of root pieces (a minimum of 300 root pieces were examined) and A is the arbuscule abundance and gives an estimation of the arbuscule richness in the whole root system.
Easily extractable glomalin (EEG) was extracted from 1 g of rhizosphere soil subsamples with citrate buffers [49, 51]. The extract from each replicate was pooled and centrifuged to remove soil particles, and protein in the supernatant was analyzed using a Bradford assay with bovine serum albumin as the standard [51].
Secondary Metabolites and Enzymatic Activities
Hydrogen peroxide (H202) concentration was determined as described by Brennan and Frenkel [12]. The concentration of the H202 in the extracts was determined by comparing the absorbance against a standard curve representing titanium-H202 level of the peroxides formed, as compared to untreated at 415 nm.
Extraction and quantification of ascorbate and glutathione, was performed following the method of Law et al. [25]. The extract, common for both metabolites, was obtained by macerating 0.5 g of fresh material (leaves) with 5 mL of metaphosphoric acid 5% (w/v). The extract was then centrifuged at 22,500 g during 15 min at 4°C. The ascorbate content was determined by means of reaction formed by 0.2 mL of supernatant, 0.5 mL of zodiac phosphate buffer 150 mM, pH 7.5, and 0.1 mL of DTT 10 mM. Tubes were shaken and incubated in the dark 10 min at room temperature. After that, 0.1 mL of N-ethylmaleimide 5% (w/v), 0.4 mL of trifluoroacetic acid 10% (v/v), 0.4 mL of 2,2-bipiridil 4% (w/v) in ethanol 70% (v/v) and 0.2 mL of FeCl3 3% (w/v) was added. The tubes were shaken and incubated in the dark at 40°C during 40 min and later absorbance was determined at 525 nm. At the same time, a calibration curve with a known ascorbate concentration was made. The results were expressed as micromole of ascorbate per gram (μmol g−1).
For the quantification of glutathione, the reaction mixture was: 50 μl of extract, 250 μl of Hepes-HCL 50 mM, pH 7.6, that contained 330 mM of betaine, and 150 μl of sulfosalicylic acid 10% (v/v). Tubes were shaken, and an aliquot of 150 μl was taken and mixed with 700 μl of NADPH 0.3 mM and 100 μl of DTNB 6 mM; after 4 min 50 μl of glutathione reductase (10 U ml−1) was added. The reaction tubes were incubated during 10 min at room temperature. Absorbance at 412 mm was determined and compared with a reference curve of glutathione. The results were expressed as micromole of glutathione per gram (μmol g−1).
Total superoxide dismutase (SOD) activity (EC 1.15.1.1) was measured according to Beyer and Fridovich [9]; the ability of SOD to inhibit the reduction of nitroblue tetrazolium (NBT) by superoxide radicals generated photochemically. One unit of SOD was defined as the amount of enzyme required to inhibit the reduction rate of NBT by 50% at 25°C. CAT activity (EC 1.11.1.6) was measured as described by Aebi [1]. Consumption of H2O2 (extinction coefficient of 39.6 mM−1 cm−1) at 240 nm for 1 min was monitored. The reaction mixture consisted of 50 mM phosphate buffer (pH 7.0) containing 10 mM H2O2 and 100 μl of enzyme extract in a 2-mL volume. Glutathione reductase (GR) activity, (EC 1.20.4.2.) was estimated measuring the decrease of 340 nm at 25°C as a result of the oxidation of NADPH [17]. The reaction mixture (1 ml) contained 0.1 M HEPES-NaOH 100 mM (pH 7.8), 1 mM EDTA, 3 mM MgCl2, 0.5 mM oxidized glutathione, 150 μl enzyme extract, and 0.2 mM NADPH was added and mixed thoroughly to begin the reaction. The results were expressed in micromole (μmol) NADPH oxidized per gram (g−1) PF min−1, and the activity was calculated starting from the initial speed of reaction and the molar extinction coefficient of NADH (ε 340 = 6.22 mM−1 cm−1).
Statistical Analyses
The data were subjected to analysis of variance (ANOVA), Duncan’s multiple-range test [18]. Percentage values were ARC-sine-transformed before statistical analysis.
Results
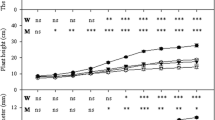
Mycorrhizal plants colonized, particularly those colonized by autochthonous strain, showed a root and shoot biomass enhancement compared to plants colonized by reference allochthonous strains or autochthonous G. mosseae (Fig. 1). The colonization by the autochthonous strains produced greater shoot and root biomass than the colonization by allochthonous strains, irrespective of fungal strain used. The magnitude of the growth response between plants colonized by autochthonous G. mosseae and allochthonous strain ranged from 62% (shoot) to 100% (root). Differences in plant biomass were lower between the G. intraradices-colonized plants as affected by the strain and the highest differences were found in root development (by 35% in weight and by 87% in length). Similarly, the root/shoot ratio was the highest in plants colonized by the autochthonous strains.
Shoot, root, shoot/root (ratio), and root length (cm) of mycorrhizal lavender plant inoculated with reference allochthonous AM fungi (G. mosseae [RM] or G. intraradices [RI]) or with autochthonous G mosseae (AM) or G. intraradices (AI) after 6 months under drought conditions. Means followed by the same letter are not significantly different (P < 0.05) as determined by Duncan’s multiple-range test (n = 5).
The highest accumulation of extraradical mycelium (as glomalin) was found in the rhizosphere of roots colonized by autochthonous fungus. Autochthonous G. intraradices was the fungus that produced the highest amount of this fungal glycoprotein. Differences in glomalin concentration were of 49% (between G. intraradices strains) and 18% (between G. mosseae strains) (Fig. 2). Vesicle formation was the lowest in AM roots colonized by the less drought-adapted endophytes. In fact, autochthonous strains formed 75% (G. intraradices) and 52% (G. mosseae) more vesicles than the corresponding allochthonous strains.
Vesicle formation and glomalin (mg g−1) on mycorrhizal lavender plant inoculated with reference allochthonous AM fungi (G. mosseae [RM] or G. intraradices [RI]) or with autochthonous G. mosseae (AM) or G. intraradices (AI) after 6 months under drought conditions. Means followed by the same letter are not significantly different (P < 0.05) as determined by Duncan’s multiple-range test (n = 5).
The effectiveness of the autochthonous mycorrhizal fungi (AI and AM) in increasing shoot and root water content was greater than by reference fungi (RI or RM); (Fig. 3).
Shoot and root water content (%), nitrogen and potassium content on lavender plant inoculated with reference allochthonous AM fungi (G. mosseae [RM] or G. intraradices [RI]) and with autochthonous G. mosseae (AM) or G. intraradices (AI) after 6 months under drought conditions. Means followed by the same letter are not significantly different (P < 0.05) as determined by Duncan’s multiple-range test (n = 5).
Shoot N and K content displayed similar trends to those described for plant growth. The contents of these nutrients in lavender shoot and root were particularly enhanced by autochthonous strains. The biggest difference in N and K plant content was observed between lavender plants colonized by each one of the two G. mosseae strains.
Mycorrhizal colonization showing SDH and ALP-ase activities by autochthonous strains was greater than by reference strains (Fig. 4). The arbuscular structures showing SDH activity were the highest in root colonized by autochthonous G. intraradices strain (Fig. 5). Nevertheless, G. intraradices produced a higher colonization rate, vitality (SDH) and activity (ALP-ase) compared to G. mosseae. The highest colonizing ability by G. intraradices was related to the greatest proportion of arbuscules (A) formed. Roots colonized by this fungus, particularly by the autochthonous drought-adapted strain, showed the greatest proportion of AM intraradical structures (M and A), vitality, and activity.
Root colonization in lavender plant inoculated with reference allochthonous AM fungi (G. mosseae [RM] or G. intraradices [RI]) or with autochthonous G. mosseae (AM) or G. intraradices (AI) after 6 months under drought conditions. AM values measured (%) were: M (colonization intensity or amount of fungal structures colonizing 100 cm of root pieces) after trypan blue (TB), succinate dehydrogenase (SDH), or alkaline phosphatase (ALP) staining. Means followed by the same letter are not significantly different (P < 0.05) as determined by Duncan’s multiple-range test (n = 5).
Root colonization in lavender plant inoculated with reference allochthonous AM fungi (G. mosseae [RM] or G. intraradices [RI]) and with autochthonous G. mosseae (AM) or G. intraradices (AI) after 6 months under drought stress. AM values measured (%) were: A (arbuscules abundance) in the whole root system after trypan blue (TB), succinate dehydrogenase (SDH), or alkaline phosphatase (ALP) staining. Means followed by the same letter are not significantly different (P < 0.05) as determined by Duncan’s multiple-range test (n = 5).
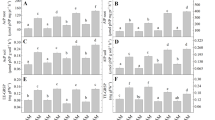
Results showed a high contribution of the fungal partner in the variability of H2O2, glutathione, and ascorbate production (Fig. 6). The lowest accumulation of such compounds was seen in plants colonized by both G. intraradices strains and the highest in those colonized by reference G. mosseae. The highest differences in the production of such compounds were found in colonized lavender by G. mosseae, autochthonous and reference strains, and these were: 108% (glutathione), 26% (ascorbate), and 43% (hydrogen peroxide). The difference between strains was more evident for glutathione. Thus, it appeared to be particularly related to the degree of drought-stress tolerance. In fact, these results show that the accumulation of antioxidants varied according to the adaptation and origin of the colonizing fungus. The contribution of the mycorrhizal fungus used in inoculation was important in affecting the production of these metabolic compounds under water stress. Nevertheless, SOD and CAT antioxidant activities were not affected by the AM fungus (Fig. 7). All of them (particularly CAT) showed similar values irrespective of the fungal strain involved. In addition, a small difference (18%) was observed in SOD activity between plants colonized by autochthonous G. intraradices and allochthonous G. mosseae (the most and less efficient AM fungi), whereas the difference in GR activity between these both treated plants was 85%.
Glutathione, ascorbate and H2O2 contents in lavender plants inoculated with reference allochthonous AM fungi (G. mosseae [RM] or G. intraradices [RI]) or with autochthonous G. mosseae (AM) or G. intraradices (AI) after 6 months under drought conditions. Means followed by the same letter are not significantly different (P < 0.05) as determined by Duncan’s multiple-range test (n = 5).
Catalase, superoxide dismutase, and glutathione reductase activities in lavender plant inoculated with reference allochthonous AM fungi (G. mosseae [RM] or G. intraradices [RI]) and with the autochthonous G. mosseae (AM) or G. intraradices (AI) after 6 months under drought conditions. Means followed by the same letter are not significantly different (P < 0.05) as determined by Duncan’s multiple-range test (n = 5).
The effect of fungal colonization on antioxidant enzymes activities differed between analyzed enzymes. In plants colonized by autochthonous G. intraradices, the increase in GR and decreased in glutathione might be related with the symbiotic effectiveness under such drought conditions.
The antioxidative induction was negatively correlated with shoot and root biomass production and mycorrhizal development. The inoculation by autochthonous fungi caused not only a greater AM colonization but also increased the physiological and metabolic status of the symbiotic development.
Discussion
The results of this study showed that the symbiotic effectiveness varied depending on the ecological adaptation and origin of the AM fungi used. Both autochthonous strains were more effective in terms of shoot and root growth, water content and nutrients uptake by lavender plants in the drought-stressed soil than similar less drought-adapted allochthonous strains used as references. The selection of effective fungal strains for inoculation could lead to the enhancement of lavender establishment in drought soils. An important differential mycorrhizal effect of the autochthonous isolates compared to the allochthonous strains was the highest stimulation of root biomass (weight) and root length and the highest proportion of N and K allocated in root tissues. These effects and the water content are indicative of drought tolerance. In a previous study, the autochthonous fungal strain G. intraradices showed the highest effectiveness on shoot and root biomass production under drought conditions when associated with the drought-adapted plant Retama sphaerocarpe and increased water transport in terms of relative plant water uptake (%) and volumetric soil moisture as previously reported [27]. The results of the AM effect on plant water content were concordant with those reported by Koide [23]. Autochthonous G. intraradices might increase water uptake by an improvement of root conductivity to water flow and/or via extraradical mycelium being able to transport water to the mycorrhizal root [13, 14, 38]. According to these results, AM extraradical mycelium, determined as the amount of glomalin, was the highest in the rhizosphere soil from autochthonous G. intraradices-colonized plants having 49% more glomalin than the rhizosphere soil from allochthonous reference strain. Glomalin as mycelial component is used as an index of fungal extraradical development. As well as binding agent, it seems to play a principal role in soil stabilization, and it may increase the level of stable aggregate because this glycoprotein acts as an insoluble glue to stabilize aggregates being effective in increasing soil structural stability and soil water availability [50]. In fact, autochthonous G. intraradices seems to be the most suitable fungus in improving soil aggregation that facilitates water infiltration, which is important in maintaining soil porosity and providing adequate oxygen supply to roots. The two autochthonous AM species exhibited significantly greater extraradical (glomalin) mycorrhizal development and plant water uptake than the two allochthonous AM isolates from collection. In concordance with these results, the greatest development of intraradical mycelium, fungal activity, and vesicular-arbuscular formations by autochthonous AM fungi were produced. The arbuscular richness (A) shows the activity of this symbiosis and is related to the nutrients and water acquired by the host plant via AM mycelium.
Several mechanisms can explain the following finding: the lowest oxidative damage and the greatest N, K, water content, and plant biomass found in lavender plants colonized by autochthonous fungal strains. The alleviation of oxidative damage is an important mechanism involved in the protective mechanisms of AM-colonized plants by selected fungi against water limitation. The efficiency of autochthonous G. mosseae in increasing plant growth, water content and nutrition under drought conditions suggests that native AM strains, isolates from dry site, promoted particular strategies of stress avoidance in colonized lavender plants when subjected to water limitation. In fact, the highest K uptake by plants colonized by particular AM fungus may play a key role in osmoregulation in lavender. K is important in controlling the water regime and as osmotic solute, is able to maintain a high-tissue water level under drought conditions [43]. Reference G. mosseae was the strain most sensitive to this stress and exhibited the lowest tolerance mechanisms to drought. It is well known that nitrogenous compounds (glycine, proline, and betaines) are implicated in drought resistance of plants as osmotic constituents [35]. Autochthonous G. mosseae leads to a lower drought-induced oxidative stress than the allochthonous strain because of the primary drought-avoidance mechanisms such as higher water, N, and K acquisition as well as to the ability of extraradical hyphae to take up water and nutrient from less accessible sources [39].
As the results showed, plants colonized by autochthonous AM fungi have a lower amount of H2O2, glutathione, and ascorbate accumulated than the corresponding drought-sensitive allochthonous fungal species. This may be considered as an indication of drought-avoidance mechanisms by autochthonous species as expected.
Antioxidative plant defense was more assured by autochthonous G. intraradices colonization as indicated by the reduced glutathione, catalase, and H2O2 concentration in lavender tissues. The indirect relation between the production of these compounds and plant tolerance resulted in a stronger root and shoot enhancement compared to plants colonized by reference strain. The development of this fungus that showed greater SDH or ALP-ase activities in AM mycelium and arbuscular production, indicating a higher mycorrhizal vitality, may also be related to plant drought tolerance.
Autochthonous G. intraradices helps the lavender plants to a higher extent under these experimental conditions. The antioxidative response in these plants seem to be mainly assured by the H2O2, glutathione and ascorbate content than by antioxidative enzymatic activities SOD, CAT, and GR measured in this article. Water stress seems not to be compensated in reference G. mosseae, and plant cells start using organic compounds at the expense of growth [30]. Other mechanisms developed in AM plants including ion transport, compartmentalization and oxidative protection, which may also explain the different resistance shown when plants were colonized by AM fungi more adapted or less adapted to drought conditions.
Hydrogen peroxide concentration is an important parameter index of many degenerative reactions associated with several stresses [33]. It has the ability to initiate reactions resulting in the production of hydroxyl radicals that provoke oxidative damage to biomolecules [10]. H2O2 is produced by several enzyme systems [31]. In some circumstances, the signaling potential and destructive power of reactive oxygen species (as H2O2) are defense mechanism [20, 26]. H2O2 is a strong oxidant that oxidizes tiol groups. In addition, glutathione reductase plays a relevant role in plant protection against biotic or abiotic stress [3]. An enhancement in this enzymatic activity has been associated with many processes such as higher protection of ascorbate and glutathione pools against stress, lower sensitivity to photo inhibition, and decreased foliar damage caused by the stress [21].
The induction of H2O2, glutathione and ascorbate by water stress considered as biochemical marker of tolerance and their protective role was widely reported [15, 33]. Results from this study suggest that the consistently lower H2O2, glutathione and ascorbate accumulation in plants colonized by autochthonous AM fungi is an indication of a lower oxidative damage to biomolecules.
The autochthonous AM fungal isolates the most appropriate fungi to be used for inoculation of plants growing under drought stress conditions. The autochthonous strains increased plant biomass concomitantly with an increase in N, K and water content and a decline in the antioxidant compounds having an important role in plant protection and metabolic functioning under water deficit.
References
Aebi, H (1984) Catalase in vitro. In: Packer, L (Ed.) Methods in Enzymology, vol 105. Oxygen Radicals in Biological Systems, Academic Press, London, pp 121–126
Allen, EB, Allen, MF (1986) Water relations of xeric grasses in the field: interactions of mycorrhizas and competition. New Phytol 104: 559–571
Aono, M, Kubo, A, Saji, H, Tanaka, K, Kondo, N (1993) Enhanced tolerance to photooxidative stress of transgenic Nicotiana tabacum with high chloroplastic glutathione-reductase activity. Plant Cell Physiol 34: 129–135
Augé, RM (2001) Water relations, drought and vesicular–arbuscular mycorrhizal symbiosis. Mycorrhiza 11: 3–42
Azcón, R, Barea, JM (1997) Mycorrhizal dependency of a representative plant species in mediterranean shrublands (Lavandula spica) as a key factor to its use for revegetation strategies in desertification-threatened areas. Appl Soil Ecol 7:83–92
Barea, JM, Azcón, R, Azcón-Aguilar, C (1992) Vesicular-arbuscular mycorrhizal fungi in nitrogen-fixing systems. In: Norris, JR, Read, DJ, Varma, A (Eds.) Methods in Microbiology, vol 24. Techniques for the Study of Mycorrhizae, Academic Press, London, pp 391–416
Barea, JM, Azcón, R, Azcón-Aguilar, C (2002) Mycorrhizosphere interactions to improve plant fitness and soil quality. Antonie Van Leeuwenhoek 81: 343–351
Barea, JM, Jeffries, P (1995) Arbuscular mycorrhizas in sustainable soil plant systems. In: Varma, A, Hock, B (Eds.) Mycorrhiza: Structure, Function, Molecular Biology and Biotechnology, Springer-Verlag, Heidelberg, pp 521–559
Beyer, WF, Fridovich, I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161: 559–566
Bowler, C, Vanmontagu, M, Inze, D (1992) Superoxide-dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43: 83–116
Bremner, JM (1996) Nitrogen total. In: Sparks, DL (Ed.) Methods of Soil Analysis. Part 3: Chemical Methods, Soil Science Society of America, American Society of Agronomy, Madison, USA, pp 1085–1121
Brennan, T, Frenkel, C (1977) Involvement of hydrogen-peroxide in regulation of senescence in pear. Plant Physiol 59: 411–416
Bryla, DR, Duniway, JM (1997) Growth, phosphorus uptake, and water relations of safflower and wheat infected with an arbuscular mycorrhizal fungus. New Phytol 136: 581–590
Bryla, DR, Duniway, JM (1997) Water uptake by safflower and wheat roots infected with arbuscular mycorrhizal fungi. New Phytol 136: 591–601
Burrit, DJ, Larkindale, J, Hurd, CL (2002). Antioxidant metabolism in the intertidal red seaweed stictosiphonia arbuscular following desiccation. Planta 215: 829–838
Caravaca, F, Barea, JM, Palenzuela, J, Figueroa, D, Alguacil, MD, Roldan, A (2002) Establishment of shrubs species in a degraded semiarid site after inoculation with native or allochtohonous arbuscular mycorrhizal fungi. Appl Soil Ecol 22: 103–111
Carlberg, I, Mannervik, B (1985) Glutathione reductase. Methods Enzymol 113: 484–489
Duncan, DB (1955) Multiple range and multiple F tests. Biometrics 11: 1–42
Essington, ME (2004) Soil and Water Chemistry: an Integrative Approach. CRC Press, Boca Raton, Florida
Foyer, CH, López-Delgado, H, Dat, JF, Scott, IM (1997) Hydrogen peroxide and glutathiones-associated mechanisms of acclimatory stress tolerance and signalling. Physiol Plant 100: 241–254
Foyer, CH, Souriau, N, Perret, S, Lelandais, M, Kunert, KJ, Pruvost, C, Jouanin, L (1995) Overexpression of glutathione-reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photoinhibition in poplar trees. Plant Physiol 109: 1047–1057
Goicoechea, N, Szalai, G, Antolín, MC, Sánchez-Díaz, M, Paldi, E (1998) Influence of arbuscular mycorrhizar and Rhizobium on free polyamines and proline levels in water-stressed alfalfa. J Plant Physiol 153: 706–711
Koide, RT (1993) Physiology of the mycorrhizal plant. Adv Plant Pathol 9: 33–54
Lachica, M, Aguilar, A, Yañez, J (1973) Análisis foliar, métodos utilizados en la Estación Experimental del Zaidín. An Edafol Agrobiol 32: 1033–1047
Law, MY, Charles, SA, Halliwell, B (1992) Glutathione and ascorbic acid in spinach (Spicacea oleracea) choloplast. The effect of hydrogen peroxide and Paraquat. Biochem J 210: 899–903
Levine, A, Tenhaken, R, Dixon, R, Lamb, C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79: 583–593
Marulanda, A, Barea, JM, Azcón, R (2006) An indigenous drought-tolerant strain of Glomus intraradices associated with a native bacterium improves water transport and root development in Retama sphaerocarpa. Microb Ecol 52:670–678
Marulanda, A, Azcón, R, Ruíz-Lozano, JM (2003) Contribution of six arbuscular mycorrhizal fungal isolates to water uptake by Lactuca sativa plants under drought stress. Physiol Plant 119: 526–533
Monzón, A, Azcón, R (1996) Relevance of mycorrhizal fungal origin and host plant genotype to inducing growth and nutrient uptake in Medicago species. Agric Ecosyst Environ 60: 9–15
Niu, DK, Wang, MG, Wang, YF (1997) Plant cellular osmotic. Acta Biotheor 45: 161–169
Noctor, G, Foyer, CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49: 249–279
Phillips, JM, Hayman, DS (1970) Improved procedure of clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55: 159–161
Porcel, R, Barea, JM, Ruíz-Lozano, JM (2003) Antioxidant activities in mycorrhizal soybean under drought stress and their possible relationship to the process of nodule senescence. New Phytol 157: 135–143
Requena, N, Jeffries, P, Barea, JM (1996) Assessment of natural mycorrhizal potential in a desertified semiarid ecosystem. Appl Environ Microbiol 62: 842–847
Rontein, D, Basset, G, Hanson, AD (2002) Metabolic engineering of osmoprotectant accumulation in plants. Metab Eng 4: 49–56
Roth, CH, Malicki, MA, Plagge, R (1992) Empirical evaluation of the relationship between soil dielectric constant and volumetric water content as the basis for calibrating soil moisture measurements. J Soil Sci 43: 1–13
Ruíz-Lozano, JM (2003) Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza 13: 309–317
Ruíz-Lozano, JM, Azcón, R (1995) Hyphal contribution to water uptake in mycorrhizal plants as affected by the fungal species and water status. Physiol Plant 95: 472–478
Ruíz-Lozano, JM, Azcón, R (1996) Mycorrhizal colonization and drought stress as factors affecting nitrate reductase activity in lettuce plants. Agric Ecosyst Environ 60: 175–181
Ruíz-Lozano, JM, Azcón, R, Gómez, M (1995) Effects of arbuscular-mycorrhizal Glomus species on drought tolerance: physiological and nutritional plant responses. Appl Environ Microbiol 61: 456–460
Smith, SE, Gianinazzi-Pearson, V (1990) Phosphate uptake and arbuscular activity in mycorrhizal Allium cepa L. Effects of photon irradiance and phosphate nutrition. Aust J Plant Physiol 17: 177–188
Sumner, ME, Miller, WP (2007) Cation exchange capacity and exchange coefficients. In: Sparks, DL (Ed.) Methods of Soil Analysis. Part 3: Chemical Methods, Soil Science Society of America, American Society of Agronomy, Madison, USA, pp 1201–1229
Thomas, HM, Morgan, WG, Humphreys, MW (2003) Designing grasses with a future—combining the attributes of Lolium and Festuca. Euphytica 133: 19–26
Tisserant, B, Gianinazzi-Pearson, V, Gianinazzi, S, Gollotte, A (1993) In Planta histochemical staining of fungal alkaline phosphatase activity for analysis of efficient arbuscular mycorrhizal infections. Mycol Res 97: 245–250
Trouvelot, A, Fardeau, JC, Plenchette, C, Gianinazzi, S, Gianinazzi-Pearson, V (1986) Nutritional balance and symbiotic expression in mycorrhizal wheat. Physiol Veg 24: 300
Vilariño, A, Arines, J (1990) An instrumental modification of Gerdeman and Nicolson’s method for extracting VAM fungal spores from soil samples. Plant Soil 121: 211–215
Vivas, A, Azcón, R, Biró, B, Barea, JM, Ruíz-Lozano, JM (2003) Influence of bacterial strains isolated from lead-polluted soil and their interactions with arbuscular mycorrhizae on the growth of Trifolium pratense L. under lead toxicity. Can J Microbiol 49: 577–588
White, I, Knight, J.H., Zegelin, SJ, Topp, GC (1994) Comments to “Considerations on the use of time-domain reflectometry (TDR) for measuring soil water content” by WR Whalley. J Soil Sci 45: 503–508
Wright, SF, Franckee-Snyder, M, Morton, M, Upadhyaya, A (1998) Time-course study and partial characterization of a protein on arbuscular mycorrhizal hyphae during active colonization of roots. Plant Soil 181: 193–203
Wright, SF, Nichols, KA, Schmidt, WF (2006) Comparison of efficacy of three extractants to solubilize glomalin on hyphae and soil. Chemosphere 64(7): 1219–1224
Wright, SF, Upadhyaya, A (1996) Extraction of an abundant and unusual protein from soil and comparison with hyphal protein of arbuscular mycorrhizal fungi. Soil Sci 161: 1–12
Acknowledgments
This study was supported by GLO FEDER-National Plan (REN2003-00968). A. M. acknowledges AECI for financial support through a MAE Fellowship. We thank Dra. C. Lluch Plá and her group for the help in the determination of antioxidant activities, C. Cano who give inoculants of fungi AM from de E.E.Z collection and R. Aroca, R. Blanco, and F. Jahromi for correcting the English text.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marulanda, A., Porcel, R., Barea, J.M. et al. Drought Tolerance and Antioxidant Activities in Lavender Plants Colonized by Native Drought-tolerant or Drought-sensitive Glomus Species. Microb Ecol 54, 543–552 (2007). https://doi.org/10.1007/s00248-007-9237-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-007-9237-y