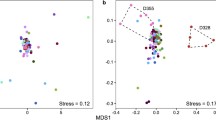
Abstract
Microbial function, composition, and distribution play a fundamental role in ecosystem ecology. The interaction between desert plants and their associated microbes is expected to greatly affect their response to changes in this harsh environment. Using comparative analyses, we studied the impact of three desert shrubs, Atriplex halimus (A), Artemisia herba-alba (AHA), and Hammada scoparia (HS), on soil- and leaf-associated microbial communities. DNA extracted from the leaf surface and soil samples collected beneath the shrubs were used to study associated microbial diversity using a sequencing survey of variable regions of bacterial 16S rRNA and fungal ribosomal internal transcribed spacer (ITS1). We found that the composition of bacterial and fungal orders is plant-type-specific, indicating that each plant type provides a suitable and unique microenvironment. The different adaptive ecophysiological properties of the three plant species and the differential effect on their associated microbial composition point to the role of adaptation in the shaping of microbial diversity. Overall, our findings suggest a link between plant ecophysiological adaptation as a “temporary host” and the biotic-community parameters in extreme xeric environments.





Similar content being viewed by others
References
Offer ZY, Steinberger Y (1994) Some data on elemental composition of airborne particulate matter in the northern Negev Desert, Israel. Environ Conserv 21:342–346
Acosta-Martinez V, Van Pelt S, Moore-Kucera J, Baddock MC, Zobeck TM (2015) Microbiology of wind-eroded sediments: current knowledge and future research directions. Aeolian Res 18:99–113
Zaady E, Ben-David EA, Sher Y, Tzirkin R, Nejidat A (2010) Inferring biological soil crust successional stage using combined PLFA, DGGE, physical and biophysiological analyses. Soil Biol Biochem 42:842–849. doi:10.1016/j.soilbio.2010.02.002
Goudie AS (2014) Desert dust and human health disorders. Environ Int 63:101–113
Fedorov DN, Doronina NV, Trotsenko YA (2011) Phytosymbiosis of aerobic methylobacteria: new facts and views. Microbiology 80:443–454
Reed SC, Townsend AR, Cleveland CC, Nemergut DR (2010) Microbial community shifts influence patterns in tropical forest nitrogen fixation. Oecologia 164:521–531
Vorholt JA (2012) Microbial life in the phyllosphere. Nat Rev Microbiol 10:828–840
Beattie GA, Lindow SE (1994) Comparison of the behavior of epiphytic fitness mutants of Pseudomonas syringae under controlled and field conditions. Appl Environ Microbiol 60:3799–3808
Wilson M, Hirano SS, Lindow SE (1999) Location and survival of leaf-associated bacteria in relation to pathogenicity and potential for growth within the leaf. Appl Environ Microbiol 65:1435–1443
Pagaling E, Wang H, Venables M, Wallace A, Grant WD, Cowan DA, Jones BE, Ma Y, Ventosa A, Heaphy S (2009) Microbial biogeography of six salt lakes in Inner Mongolia, China, and a salt lake in Argentina. Appl Environ Microbiol 75:5750–5760. doi:10.1128/AEM.00040-09
Evenari ME, Shanan L, Tadmor W (1982) The Negev: the challenge of a desert, 2nd edn. Harvard University Press, Cambridge, p 345
Guenaoui C, Gorai M, Smiti S, Neffati M (2008) Biochemical and physiological changes in Artemisia herba-alba plants under water stress conditions. Middle-East J Sci Res 3:156–163
Martirosyan V, Steinberger Y (2014) Microbial functional diversity in the phyllosphere and laimosphere of different desert plants. J Arid Environ 107:26–33. doi:10.1016/j.jaridenv.2014.04.002
Whitford WG (2002) Ecology of desert systems. Academic Press, New York, p 343
Qvit-Raz N, Jurkevitch E, Belkin S (2008) Drop-size soda lakes: transient microbial habitats on a salt-secreting desert tree. Genetics 178:1615–1622. doi:10.1534/genetics.107.082164
Finkel OM, Burch AY, Lindow SE, Post AF, Belkin S (2011) Geographical location determines the population structure in phyllosphere microbial communities of a salt-excreting desert tree. Appl Environ Microbiol 77:7647–7655. doi:10.1128/aem.05565-11
Finkel OM, Burch AY, Elad T, Huse SM, Lindow SE, Post AF, Belkin S (2012) Distance-decay relationships partially determine diversity patterns of phyllosphere bacteria on Tamarix trees across the Sonoran Desert. Appl Environ Microbiol 78:6187–6193
Muller T, Ruppel S (2014) Progress in cultivation-independent phyllosphere microbiology. FEMS Microbiol Ecol 87:2–17
Bowers RM, Sullivan AP, Costello EK, Collett JL, Knight R, Fierer N (2011) Sources of bacteria in outdoor air across cities in the Midwestern United States. Appl Environ Microbiol 77:6350–6356. doi:10.1128/Aem.05498-11
Lozupone CA, Knight R (2007) Global patterns in bacterial diversity. Proc Natl Acad Sci U S A 104:11436–11440
Rothberg JM, Hinz W, Rearick TM, Schultz J, Mileski W, Davey M, Leamon JH, Johnson K, Milgrew MJ, Edwards M, Hoon J, Simons JF, Marran D, Myers JW, Davidson JF, Branting A, Nobile JR, Puc BP, Light D, Clark TA, Huber M, Branciforte JT, Stoner IB, Cawley SE, Lyons M, Fu YT, Homer N, Sedova M, Miao X, Reed B, Sabina J, Feierstein E, Schorn M, Alanjary M, Dimalanta E, Dressman D, Kasinskas R, Sokolsky T, Fidanza JA, Namsaraev E, McKernan KJ, Williams A, Roth GT, Bustillo J (2011) An integrated semiconductor device enabling non-optical genome sequencing. Nature 475:348–352
Knief C (2014) Analysis of plant microbe interactions in the era of next generation sequencing technologies. Front Plant Sci 5:216. doi:10.3389/fpls.2014.00216
Barriuso J, Valverde JR, Mellado RP (2011) Estimation of bacterial diversity using next generation sequencing of 16S rDNA: a comparison of different workflows. BMC Bioinformatics 12:473. doi:10.1186/1471-2105-12-473
Unterseher M, Jumpponen A, Opik M, Tedersoo L, Moora M, Dormann CF, Schnittler M (2011) Species abundance distributions and richness estimations in fungal metagenomics—lessons learned from community ecology. Mol Ecol 20:275–285
Guo SZ, Lu ZM, Kang GY, Chen Z, Luo H (2012) A tree-structured deterministic small-world network. IEICE Trans Inform Syst E95d:1536–1538. doi:10.1587/transinf.E95.D.1536
Martirosyan V, Steinberger Y, Doniger T, Wachtel C, Miller G (2014) Structural and functional microbial diversity determined by shrubs ecophysiological adaptation in the Negev Desert. The 99th Annual Meeting of the Ecological Society of America, August 10–15, 2014, Sacramento, CA
Barness G, Zaragoza S, Shmueli I, Steinberger Y (2009) Vertical distribution of a soil microbial community as affected by plant ecophysiological adaptation in a desert system. Microb Ecol 57:36–49. doi:10.1007/s00248-008-9396-5
Berg N, Steinberger Y (2008) Role of perennial plants in determining the activity of the microbial community in the Negev Desert ecosystem. Soil Biol Biochem 40:2686–2695. doi:10.1016/j.soilbio.2008.07.019
Mohamed AEHH, El-Sayed MA, Hegazy ME, Helaly SE, Esmail AM, Mohamed NS (2010) Chemical constituents and biological activities of Artemisia herba-alba. Rec Nat Prod 4:1–25
Muyzer G, Ramsing NB (1995) Molecular methods to study the organization of microbial communities. Water Sci Technol 32:1–9
Vasileiadis S, Puglisi E, Arena M, Cappa F, Cocconcelli PS, Trevisan M (2012) Soil bacterial diversity screening using single 16S rRNA gene V regions coupled with multi-million read generating sequencing technologies. PLoS ONE 7:e42671
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118. doi:10.1111/j.1365-294X.1993.tb00005.x
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Smith DP, Peay KG (2014) Sequence depth, not PCR replication, improves ecological inference from next generation DNA sequencing. PLoS ONE 9:e90234
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R (2011) Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A 108:4516–4522. doi:10.1073/pnas.1000080107
Knight R, Maxwell P, Birmingham A, Carnes J, Caporaso JG, Easton BC, Eaton M, Hamady M, Lindsay H, Liu ZZ, Lozupone C, McDonald D, Robeson M, Sammut R, Smit S, Wakefield MJ, Widmann J, Wikman S, Wilson S, Ying H, Huttley GA (2007) PyCogent: a toolkit for making sense from sequence. Genome Biol 8:R171
McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P (2012) An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J 6:610–618
Koljalg U, Nilsson RH, Abarenkov K, Tedersoo L, Taylor AFS, Bahram M, Bates ST, Bruns TD, Bengtsson-Palme J, Callaghan TM, Douglas B, Drenkhan T, Eberhardt U, Duenas M, Grebenc T, Griffith GW, Hartmann M, Kirk PM, Kohout P, Larsson E, Lindahl BD, Luecking R, Martin MP, Matheny PB, Nguyen NH, Niskanen T, Oja J, Peay KG, Peintner U, Peterson M, Poldmaa K, Saag L, Saar I, Schuessler A, Scott JA, Senes C, Smith ME, Suija A, Taylor DL, Telleria MT, Weiss M, Larsson KH (2013) Towards a unified paradigm for sequence-based identification of fungi. Mol Ecol 22:5271–5277
Berg N, Steinberger Y (2010) Are biological effects of desert shrubs more important than physical effects on soil microorganisms? Microb Ecol 59:121–129. doi:10.1007/s00248-009-9599-4
Schrempf H (2013) Actinobacteria within soils: capacities for mutualism, symbiosis and pathogenesis. FEMS Microbiol Lett 342:77–78
Bulgarelli D, Schlaeppi K, Spaepen S, van Themaat EVL, Schulze-Lefert P (2013) Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol 64:807–838
Bachar A, Soares MIM, Gillor O (2012) The effect of resource islands on abundance and diversity of bacteria in arid soils. Microb Ecol 63:694–700
Yu J, Grishkan I, Steinberger Y (2013) Microfungal-community diversity in Zygophyllum dumosum and Hammada scoparia root zones in the northern Negev Desert. J Basic Microbiol 53:390–400
Lau JA, Lennon JT (2011) Evolutionary ecology of plant-microbe interactions: soil microbial structure alters selection on plant traits. New Phytol 192:215–224
Brockett BFT, Prescott CE, Grayston SJ (2012) Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol Biochem 44:9–20
Lindow SE, Brandl MT (2003) Microbiology of the phyllosphere. Appl Environ Microbiol 69:1875–1883
Fahlgren C, Hagstrom A, Nilsson D, Zweifel UL (2010) Annual variations in the diversity, viability, and origin of airborne bacteria. Appl Environ Microbiol 76:3015–3025
Whitman WB, Coleman DC, Wiebe WJ (1998) Prokaryotes: the unseen majority. Proc Natl Acad Sci U S A 95:6578–6583
Vokou D, Vareli K, Zarali E, Karamanoli K, Constantinidou HIA, Monokrousos N, Halley JM, Sainis I (2012) Exploring biodiversity in the bacterial community of the Mediterranean phyllosphere and its relationship with airborne bacteria. Microb Ecol 64:714–724
Redford AJ, Bowers RM, Knight R, Linhart Y, Fierer N (2010) The ecology of the phyllosphere: geographic and phylogenetic variability in the distribution of bacteria on tree leaves. Environ Microbiol 12:2885–2893. doi:10.1111/j.1462-2920.2010.02258.x
Kim SK, Gignoux CR, Wall JD, Lum-Jones A, Wang HS, Haiman CA, Chen GK, Henderson BE, Kolonel LN, Le Marchand L, Stram DO, Saxena R, Cheng I (2012) Population genetic structure and origins of native Hawaiians in the multiethnic cohort study. PLoS ONE 7:e47881
Knief C, Ramette A, Frances L, Alonso-Blanco C, Vorholt JA (2010) Site and plant species are important determinants of the Methylobacterium community composition in the plant phyllosphere. ISME J 4:719–728. doi:10.1038/Ismej.2010.9
van Overbeek L, van Elsas JD (2008) Effects of plant genotype and growth stage on the structure of bacterial communities associated with potato (Solanum tuberosum L.). FEMS Microbiol Ecol 64:283–296. doi:10.1111/j.1574-6941.2008.00469.x
Acknowledgments
We wish to thank Ms. Sharon Victor for her helpful comments. This study was part of a research program funded by the UNESCO/Japan Young Researchers’ Fellowship Program, 2012 (UNESCO/Keizo Obuchi Research Fellowships Program), awarded to Dr. Varsik Martirosyan, and which covered her stay in Israel. The sponsor played no role in the study design, collection, analysis, data interpretation, writing of the paper, or the decision to submit it for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Funding
This study was part of a research program funded by the UNESCO/Japan Young Researchers’ Fellowship Program, 2012 (UNESCO/Keizo Obuchi Research Fellowships Program), awarded to Dr. Varsik Martirosyan, and which covered her stay in Israel.
Rights and permissions
About this article
Cite this article
Martirosyan, V., Unc, A., Miller, G. et al. Desert Perennial Shrubs Shape the Microbial-Community Miscellany in Laimosphere and Phyllosphere Space. Microb Ecol 72, 659–668 (2016). https://doi.org/10.1007/s00248-016-0822-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-016-0822-9