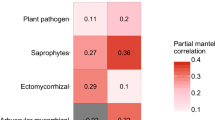
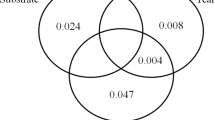
Abstract
Fungi provide essential ecosystem services and engage in a variety of symbiotic relationships with trees. In this study, we investigate the spatial relationship of trees and fungi at a community level. We characterized the spatial dynamics for above- and belowground fungi using a series of forest monitoring plots, at nested spatial scales, located in the tropical South Pacific, in Vanuatu. Fungal communities from different habitats were sampled using metagenomic analysis of the nuclear ribosomal ITS1 region. Fungal communities exhibited strong distance–decay of similarity across our entire sampling range (3–110,000 m) and also at small spatial scales (< 50 m). Unexpectedly, this pattern was inverted at an intermediate scale (3.7–26 km). At large scales (80–110 km), belowground and aboveground fungal communities responded inversely to increasing geographic distance. Aboveground fungal community turnover (beta diversity) was best explained, at all scales, by geographic distance. In contrast, belowground fungal community turnover was best explained by geographic distance at small scales and tree community composition at large scales. Fungal communities from various habitats respond differently to the influences of habitat and geographic distance. At large geographic distances (80–110 km), community turnover for aboveground fungi is better explained by spatial distance, whereas community turnover for belowground fungi is better explained by plant community turnover. Future syntheses of spatial dynamics among fungal communities must explicitly consider geographic scale to appropriately contextualize community turnover.




Similar content being viewed by others
Availability of Data and Material (Data Transparency)
The sequencing dataset analyzed during the current study is available in the NCBI Sequence Read Archive. (BioProject ID PRJNA634909). Tree community and transect data are available from Figshare (doi https://doi.org/10.6084/m9.figshare.12367475).
References
Van Der Heijden MGA, Bardgett RD, Van Straalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310. https://doi.org/10.1111/j.1461-0248.2007.01139.x
Schimann H, Bach C, Lengelle J, Louisanna E, Barantal S, Murat C, Buée M (2016) Diversity and structure of fungal communities in Neotropical rainforest soils: the effect of host recurrence. Microb Ecol 73:1–11. https://doi.org/10.1007/s00248-016-0839-0
Bagchi R, Gallery RE, Gripenberg S, Gurr SJ, Narayan L, Addis CE, Freckleton RP, Lewis OT (2014) Pathogens and insect herbivores drive rainforest plant diversity and composition. Nature 506:85–88. https://doi.org/10.1038/nature12911
Van Der Heijden MGA, Boller T, Sanders IR (1998) Different arbuscular mycorrhizal fungal species are potential. Ecology 79:2082–2091
Nacke H, Goldmann K, Schöning I, Pfeiffer B, Kaiser K, Castillo-Villamizar GA, Schrumpf M, Buscot F, Daniel R, Wubet T (2016) Fine spatial scale variation of soil microbial communities under European beech and Norway spruce. Front Microbiol 7. https://doi.org/10.3389/fmicb.2016.02067
Hawksworth DL, Lücking R (2017) Fungal Diversity Revisited : 2 . 2 to 3 . 8 Million Species:1–17. https://doi.org/10.1128/microbiolspec.FUNK-0052-2016.Correspondence
Pimm SL, Joppa LN (2015) How many plant species are there, where are they, and at what rate are they going extinct? Ann Missouri Bot Gard 100:170–176. https://doi.org/10.3417/2012018
Tobler W (1970) A computer movie simulating urban growth in the Detroit region. Econ Geogr 46:234–240
Morlon H, Chuyong G, Condit R, Hubbell S, Kenfack D, Thomas D, Valencia R, Green JL (2008) A general framework for the distance-decay of similarity in ecological communities. Ecol Lett 11:904–917. https://doi.org/10.1111/j.1461-0248.2008.01202.x
Nekola JC, White PS, Biogeography J et al (2007) Special Paper: The distance decay of similarity in biogeography and ecology the distance decay of similarity in biogeography and ecology. J Biogeogr 26:867–878
Condit R, Pitman N, Leigh EG et al (2002) Beta-diversity in tropical forest trees. Science 295(80):666–669. https://doi.org/10.1126/science.1066854
Astorga A, Oksanen J, Luoto M, Soininen J, Virtanen R, Muotka T (2012) Distance decay of similarity in freshwater communities: do macro- and microorganisms follow the same rules? Glob Ecol Biogeogr 21:365–375. https://doi.org/10.1111/j.1466-8238.2011.00681.x
Monroy F, van der Putten WH, Yergeau E, Mortimer SR, Duyts H, Bezemer TM (2012) Community patterns of soil bacteria and nematodes in relation to geographic distance. Soil Biol Biochem 45:1–7. https://doi.org/10.1016/j.soilbio.2011.10.006
Finkel OM, Burch AY, Elad T, Huse SM, Lindow SE, Post AF, Belkin S (2012) Distance-decay relationships partially determine diversity patterns of phyllosphere bacteria on Tamrix trees across the sonoran desert. Appl Environ Microbiol 78:6187–6193. https://doi.org/10.1128/AEM.00888-12
Qvit-Raz N, Finkel OM, Al-Deeb TM et al (2012) Biogeographical diversity of leaf-associated microbial communities from salt-secreting Tamarix trees of the Dead Sea region. Res Microbiol 163:142–150. https://doi.org/10.1016/j.resmic.2011.11.006
Bell T (2010) Experimental tests of the bacterial distance-decay relationship. ISME J 4:1357–1365. https://doi.org/10.1038/ismej.2010.77
Yavitt JB, Yashiro E, Cadillo-Quiroz H, Zinder SH (2012) Methanogen diversity and community composition in peatlands of the central to northern Appalachian Mountain region, North America. Biogeochemistry 109:117–131. https://doi.org/10.1007/s10533-011-9644-5
Saleem M, Pervaiz ZH, Traw MB (2015) Theories, mechanisms and patterns of microbiome species coexistence in an era of climate change. Microbiome community ecology: fundamentals and applications. Springer International Publishing, Cham, pp 13–53
Martiny JBH, Eisen JA, Penn K, Allison SD, Horner-Devine MC (2011) Drivers of bacterial beta-diversity depend on spatial scale. Proc Natl Acad Sci U S A 108:7850–7854. https://doi.org/10.1073/pnas.1016308108
Vaz ABM, Fontenla S, Rocha FS, Brandão LR, Vieira MLA, de Garcia V, Góes-Neto A, Rosa CA (2014) Fungal endophyte β-diversity associated with Myrtaceae species in an Andean Patagonian forest (Argentina) and an Atlantic forest (Brazil). Fungal Ecol 8:28–36. https://doi.org/10.1016/j.funeco.2013.12.008
Cordier T, Robin C, Capdevielle X, Desprez-Loustau ML, Vacher C (2012) Spatial variability of phyllosphere fungal assemblages: genetic distance predominates over geographic distance in a European beech stand (Fagus sylvatica). Fungal Ecol 5:509–520. https://doi.org/10.1016/j.funeco.2011.12.004
Vincent JB, Weiblen GD, May G (2016) Host associations and beta diversity of fungal endophyte communities in New Guinea rainforest trees. Mol Ecol 25:825–841. https://doi.org/10.1111/mec.13510
Oono R, Rasmussen A, Lefèvre E (2017) Distance decay relationships in foliar fungal endophytes are driven by rare taxa. Environ Microbiol 00:2794–2805. https://doi.org/10.1111/1462-2920.13799
Kadowaki K, Sato H, Yamamoto S, Tanabe AS, Hidaka A, Toju H (2014) Detection of the horizontal spatial structure of soil fungal communities in a natural forest. Popul Ecol 56:301–310. https://doi.org/10.1007/s10144-013-0424-z
Bahram M, Kõljalg U, Courty PE, Diédhiou AG, Kjøller R, Põlme S, Ryberg M, Veldre V, Tedersoo L (2013) The distance decay of similarity in communities of ectomycorrhizal fungi in different ecosystems and scales. J Ecol 101:1335–1344. https://doi.org/10.1111/1365-2745.12120
Baldrian P (2017) Forest microbiome: diversity, complexity and dynamics. FEMS Microbiol Rev 41:109–130. https://doi.org/10.1093/femsre/fuw040
Barberan A, Mcguire KL, Wolf JA et al (2015) Relating belowground microbial composition to the taxonomic, phylogenetic, and functional trait distributions of trees in a tropical forest. Ecol Lett 18:1397–1405. https://doi.org/10.1111/ele.12536
von Humboldt A, Bonpland A (1807) Essai sur la géographie des plantes
Tedersoo L, Bahram M, Põlme S et al (2014) Global diversity and geography of soil fungi. Science (80):346. https://doi.org/10.1126/science.1256688
Peay KG, Bidartondo MI, Elizabeth Arnold A (2010) Not every fungus is everywhere: scaling to the biogeography of fungal-plant interactions across roots, shoots and ecosystems. New Phytol 185:878–882
Meyer KM, Memiaghe H, Korte L, Kenfack D, Alonso A, Bohannan BJM (2018) Why do microbes exhibit weak biogeographic patterns? ISME J 12:1404–1413. https://doi.org/10.1038/s41396-018-0103-3
Mallick DIJ (1975) Development of the New Hebrides Archipelago. Philos Trans R Soc Lond Ser B Biol Sci 272:277–285. https://doi.org/10.1098/rstb.1975.0087
Mueller-Dombois D, Fosberg FR (2013) Vegetation of the tropical Pacific islands. Springer Science & Business Media
Vanuatu Meteorology and Geo-hazard Department, Australian Bureau of Meteorology, CSIRO (2015) Current and future climate of Vanuatu. Melbourne, Australia
McMurdie PJ, Holmes S (2014) Waste not, Want not: why rarefying microbiome data is inadmissible. PLoS Comput Biol 10:e1003531. https://doi.org/10.1371/journal.pcbi.1003531
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. https://doi.org/10.1186/s13059-014-0550-8
R Core Team (2019) R: A language and environment for statistical computing. Accessed 1st April 2019
McMurdie PJ, Holmes S (2013) Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PloS one 8(4):e61217
Ferrier S, Manion G, Elith J, Richardson K (2007) Using generalized dissimilarity modelling to analyse and predict patterns of beta diversity in regional biodiversity assessment. Divers Distrib 13:252–264. https://doi.org/10.1111/j.1472-4642.2007.00341.x
Fitzpatrick MC, Sanders NJ, Normand S, Svenning JC, Ferrier S, Gove AD, Dunn RR (2013) Environmental and historical imprints on beta diversity: insights from variation in rates of species turnover along gradients. Proc R Soc B Biol Sci 280:20131201–20131201. https://doi.org/10.1098/rspb.2013.1201
Robinson CH, Szaro TM, Izzo AD, Anderson IC, Parkin PI, Bruns TD (2009) Spatial distribution of fungal communities in a coastal grassland soil. Soil Biol Biochem 41:414–416. https://doi.org/10.1016/j.soilbio.2008.10.021
Peay KG, Kennedy PG, Talbot JM (2016) Dimensions of biodiversity in the earth mycobiome. Nat Rev Microbiol 14:434–447. https://doi.org/10.1038/nrmicro.2016.59
Tuomisto H, Ruokolainen K, Yli-Halla M (2003) Dispersal, environment, and floristic variation of Western Amazonian forests. Science 299(80):241–244. https://doi.org/10.1126/science.1078037
Van Der Linde S, Suz LM, Orme CDL et al (2018) Environment and host as large-scale controls of ectomycorrhizal fungi. Nature 558:243–248. https://doi.org/10.1038/s41586-018-0189-9
David AS, Seabloom EW, May G (2016) Plant host species and geographic distance affect the structure of aboveground fungal Symbiont communities, and environmental filtering affects belowground communities in a coastal dune ecosystem. Microb Ecol 71:912–926. https://doi.org/10.1007/s00248-015-0712-6
Poulin R (2003) The decay of similarity with geographical distance in parasite communities of vertebrate hosts. J Biogeogr 30:1609–1615. https://doi.org/10.1046/j.1365-2699.2003.00949.x
Kembel SW, Mueller RC (2014) Plant traits and taxonomy drive host associations in tropical Phyllosphere fungal communities. Botany. https://doi.org/10.1139/cjb-2013-0194
Thomas D, Vandegrift R, Roy BA, Hsieh HM, Ju YM (2019) Spatial patterns of fungal endophytes in a subtropical montane rainforest of northern Taiwan. Fungal Ecol 39:316–327. https://doi.org/10.1016/j.funeco.2018.12.012
Acknowledgments
The authors would like to thank the reviewers and Drs. Nicole Hynson, Tom Ranker, Nhu Nguyen, and Michael Kantar for improving this manuscript. We are also very appreciative of Presley Dovo and the Vanuatu Department of Forestry for logistical support. This project would not be possible without field support from the ever-growing network of people associated with Plants mo Pipol blong Vanuatu. We would also like to recognize the contributions of the late Philemon Ala who had been helping with Plants mo Pipol since its inception. Finally, we are grateful to the many communities of Aneityum and Tanna for their kindness, hospitality, and for sharing so much invaluable knowledge, tankyu tumas.
Funding
A.B., T.T., and A.S.A. were supported by a grant from the National Science Foundation (1555793). G.M.P. was supported by a National Science Foundation grant awarded to NYGB (1555657).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Boraks, A., Plunkett, G.M., Doro, T.M. et al. Scale-Dependent Influences of Distance and Vegetation on the Composition of Aboveground and Belowground Tropical Fungal Communities. Microb Ecol 81, 874–883 (2021). https://doi.org/10.1007/s00248-020-01608-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-020-01608-4