Abstract
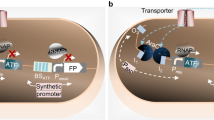
A novel uric-acid-responsive regulatory system was developed in Escherichia coli by adapting the HucR-related regulatory elements from Deinococcus radiodurans into E. coli. The induction performance of this system was compared to the performance of both the pBAD and pET systems. Our novel regulatory system was induced in a dose-dependent manner in the presence of uric acid and exhibited low basal expression in its absence. The system was characterized by a wide dynamic range of induction, being compatible with various E. coli strains and not requiring genomic modifications of the bacterial host. E. coli DH5α and DH10B were the most suitable host strains for optimal performance of this system. In conclusion, we developed a regulatory system with potential for applications in both recombinant protein expression and metabolic optimization.







Similar content being viewed by others
References
Aaron N, Milton W (1957) Enzyme induction as an all-or-none phenomenon. Proc Natl Acad Sci U S A 43:553–566
Amann E, Brosius J, Ptashne M (1983) Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene 25:167–178
Amann E, Ochs B, Abel K-J (1988) Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene 69:301–315
Baneyx F (1999) Recombinant protein expression in Escherichia coli. Curr Opin Biotechnol 10:411–421
Barrick D, Villanueba K, Childs J, Kalil R, Schneider TD, Lawrence CE, Gold L, Stormo GD (1994) Quantitative analysis of ribosome binding sites in E. coli. Nucleic Acids Res 22:1287–1295
Battista JR (1997) Against all odds: the survival strategies of Deinococcus radiodurans. Annu Rev Microbiol 51:203–224
Blazeck J, Alper HS (2013) Promoter engineering: recent advances in controlling transcription at the most fundamental level. Biotechnol J 8:46–58
Carrier TA, Keasling JD (1999) Investigating autocatalytic gene expression systems through mechanistic modeling. J Theor Biol 201:25–36
Choi YJ, Morel L, Le François T, Bourque D, Bourget L, Groleau D, Massie B, Míguez CB (2010) Novel, versatile, and tightly regulated expression system for Escherichia coli strains. Appl Environ Microbiol 76:5058–5066
Cox MM, Battista JR (2005) Deinococcus radiodurans - the consummate survivor. Nat Rev Microbiol 3:882–892
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645
Figge J, Wright C, Collins CJ, Roberts TM, Livingston DM (1988) Stringent regulation of stably integrated chloramphenicol acetyl transferase genes by E. coli lac repressor in monkey cells. Cell 52:713–722
Guan L, Liu Q, Li C, Zhang Y (2013) Development of a Fur-dependent and tightly regulated expression system in Escherichia coli for toxic protein synthesis. BMC Biotechnol 13:25–33
Guido NJ, Wang X, Adalsteinsson D, McMillen D, Hasty J, Cantor CR, Elston TC, Collins JJ (2006) A bottom-up approach to gene regulation. Nature 439:856–860
Gupta JC, Jaisani M, Pandey G, Mukherjee KJ (1999) Enhancing recombinant protein yields in Escherichia coli using the T7 system under the control of heat inducible λPL promoter. J Biotechnol 68:125–134
Guzman LM, Belin D, Carson MJ, Beckwith J (1995) Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol 177:4121–4130
Hannig G, Makrides SC (1998) Strategies for optimizing heterologous protein expression in Escherichia coli. Trends Biotechnol 16:54–60
Harley C, Reynolds RP (1987) Analysis of E. coli promoter sequences. Nucleic Acids Res 5:2343–2361
Hooper DC, Spitsin S, Kean RB, Champion JM, Dickson GM, Chaudhry I, Koprowski H (1998) Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci U S A 95:675–680
Karin H, Ruhdal JP (1998) Artificial promoter libraries for selected organisms and promoters derived from such libraries. Patent No. WO1998007846
Keasling JD (1999) Gene-expression tools for the metabolic engineering of bacteria. Trends Biotechnol 17:452–460
Khlebnikov A, Keasling JD (2002) Effect of lacY expression on homogeneity of induction from the Ptac and Ptrc promoters by natural and synthetic inducers. Biotechnol Prog 18:672–674
Lee SK, Keasling JD (2005) A propionate-inducible expression system for enteric bacteria. Appl Environ Microbiol 71:6856–6862
Lee T, Krupa R, Zhang F, Hajimorad M, Holtz W, Prasad N, Lee S, Keasling J (2011) BglBrick vectors and datasheets: a synthetic biology platform for gene expression. J Biol Eng 5:1–14
Lutz R, Bujard H (1997) Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1-I2 regulatory elements. Nucleic Acids Res 25:1203–1210
Maeda H, Fujita N, Ishihama A (2000) Competition among seven Escherichia coli σ subunits: relative binding affinities to the core RNA polymerase. Nucleic Acids Res 28:3497–3503
Nocadello S, Swennen E (2012) The new pLAI (lux regulon based auto-inducible) expression system for recombinant protein production in Escherichia coli. Microb Cell Fact 11:3–12
Noh K-H, Son J-W, Kim H-J, Oh D-K (2009) Ginsenoside compound K production from ginseng root extract by a thermostable β-glycosidase from Sulfolobus solfataricus. Biosci Biotechnol Biochem 73:316–321
Papakostas K, Frillingos S (2012) Substrate selectivity of YgfU, a uric acid transporter from Escherichia coli. J Biol Chem 287:15684–15695
Peti W, Page R (2007) Strategies to maximize heterologous protein expression in Escherichia coli with minimal cost. Protein Expr Purif 51:1–10
Samuelson J (2011) Recent developments in difficult protein expression: a guide to E. coli strains, promoters, and relevant host mutations. In: Evans JTC, Xu M-Q (eds) Heterologous gene expression in E. coli. Humana Press, New York, pp 195–209
Siegele DA, Hu JC (1997) Gene expression from plasmids containing the araBAD promoter at subsaturating inducer concentrations represents mixed populations. Proc Natl Acad Sci U S A 94:8168–8172
Stark MJR (1987) Multicopy expression vectors carrying the lac represser gene for regulated high-level expression of genes in Escherichia coli. Gene 51:255–267
Studier FW, Moffatt BA (1986) Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol 189:113–130
Tabor S (2001) Expression using the T7 RNA polymerase/promoter system. In: Ausubel FA, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (eds) Current protocols in molecular biology. Wiley, New York, pp 16.2.1–16.2.11
Wagner S, Klepsch MM, Schlegel S, Appel A, Draheim R, Tarry M, Högbom M, van Wijk KJ, Slotboom DJ, Persson JO, de Gier J-W (2008) Tuning Escherichia coli for membrane protein overexpression. Proc Natl Acad Sci U S A 105:14371–14376
Wilkinson SP, Grove A (2004) HucR, a novel uric acid-responsive member of the MarR family of transcriptional regulators from Deinococcus radiodurans. J Biol Chem 279:51442–51450
Wilkinson SP, Grove A (2005) Negative cooperativity of uric acid binding to the transcriptional regulator HucR from Deinococcus radiodurans. J Mol Biol 350:617–630
Zhang F, Carothers JM, Keasling JD (2012) Design of a dynamic sensor-regulator system for production of chemicals and fuels derived from fatty acids. Nat Biotechnol 30:354–359
Zheng L, Baumann U, Reymond J-L (2004) An efficient one-step site-directed and site-saturation mutagenesis protocol. Nucleic Acids Res 32:115–122
Acknowledgments
The authors would like to thank Mr. Ye Mao for his technical assistance in this research. This work was supported by the Ministry of Science and Technology of China Grant 2013CB734003, the National Natural Science Foundation of China Grant 21172095, and the Key Research Program of the Chinese Academy of Sciences Grant KSZD-EW-Z-015-2.
Author information
Authors and Affiliations
Corresponding author
Additional information
Chaoning Liang and Dandan Xiong contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 88 kb)
Rights and permissions
About this article
Cite this article
Liang, C., Xiong, D., Zhang, Y. et al. Development of a novel uric-acid-responsive regulatory system in Escherichia coli . Appl Microbiol Biotechnol 99, 2267–2275 (2015). https://doi.org/10.1007/s00253-014-6290-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6290-6