Abstract
Purposes
Whether low-calorie sweeteners (LCS), such as sucralose and acesulfame K, can alter glucose metabolism is uncertain, particularly given the inconsistent observations relating to insulin resistance in recent human trials. We hypothesized that these discrepancies are accounted for by the surrogate tools used to evaluate insulin resistance and that PET 18FDG, given its capacity to quantify insulin sensitivity in individual organs, would be more sensitive in identifying changes in glucose metabolism. Accordingly, we performed a comprehensive evaluation of the effects of LCS on whole-body and organ-specific glucose uptake and insulin sensitivity in a large animal model of morbid obesity.
Methods
Twenty mini-pigs with morbid obesity were fed an obesogenic diet enriched with LCS (sucralose 1 mg/kg/day and acesulfame K 0.5 mg/kg/day, LCS diet group), or without LCS (control group), for 3 months. Glucose uptake and insulin sensitivity were determined for the duodenum, liver, skeletal muscle, adipose tissue and brain using dynamic PET 18FDG scanning together with direct measurement of arterial input function. Body composition was also measured using CT imaging and energy metabolism quantified with indirect calorimetry.
Results
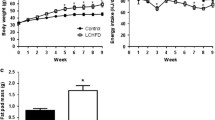
The LCS diet increased subcutaneous abdominal fat by ≈ 20% without causing weight gain, and reduced insulin clearance by ≈ 40%, while whole-body glucose uptake and insulin sensitivity were unchanged. In contrast, glucose uptake in the duodenum, liver and brain increased by 57, 66 and 29% relative to the control diet group (P < 0.05 for all), while insulin sensitivity increased by 53, 55 and 28% (P < 0.05 for all), respectively. In the brain, glucose uptake increased significantly only in the frontal cortex, associated with improved metabolic connectivity towards the hippocampus and the amygdala.
Conclusions
In miniature pigs, the combination of sucralose and acesulfame K is biologically active. While not affecting whole-body insulin resistance, it increases insulin sensitivity and glucose uptake in specific tissues, mimicking the effects of obesity in the adipose tissue and in the brain.





Similar content being viewed by others
Abbreviations
- LCS:
-
Low-calorie sweeteners
- 18FDG:
-
18Fluorodeoxyglucose
- PET-CT:
-
Positron emission tomography coupled with computed tomography
- MRglu:
-
Metabolic rate for glucose utilization
- ROI:
-
Region of interest
- VOI:
-
Volume of interest
References
Pepino MY, Tiemann CD, Patterson BW, Wice BM, Klein S. Sucralose affects glycemic and hormonal responses to an oral glucose load. Diabetes Care. 2013;36:2530–5.
Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181–6.
Suez J, Korem T, Zilberman-Schapira G, Segal E, Elinav E. Non-caloric artificial sweeteners and the microbiome: findings and challenges. Gut Microbes. 2015;6:149–55.
Lertrit A, Srimachai S, Saetung S, Chanprasertyothin S, Chailurkit L-O, Areevut C, et al. Effects of sucralose on insulin and glucagon-like peptide-1 secretion in healthy subjects: A randomized, double-blind, placebo-controlled trial. Nutrition. 2018;55-56:140–5.
Romo-Romo A, Aguilar-Salinas CA, Brito-Córdova GX, Gómez-Díaz RA, Almeda-Valdes P. Sucralose decreases insulin sensitivity in healthy subjects: a randomized controlled trial. Am J Clin Nutr. 2018;108:485–91.
Bonnet F, Tavenard A, Esvan M, Laviolle B, Viltard M, Lepicard EM, et al. Consumption of a Carbonated Beverage with High-Intensity Sweeteners Has No Effect on Insulin Sensitivity and Secretion in Nondiabetic Adults. J Nutr. 2018;148:1293–9.
Hess EL, Myers EA, Swithers SE, Hedrick VE. Associations Between Nonnutritive Sweetener Intake and Metabolic Syndrome in Adults. J Am Coll Nutr. 2018:1–7.
Liang Y, Steinbach G, Maier V, Pfeiffer EF. The effect of artificial sweetener on insulin secretion. 1. The effect of acesulfame K on insulin secretion in the rat (studies in vivo). Horm Metab Res. 1987;19:233–8.
Cong WN, Wang R, Cai H, Daimon CM, Scheibye-Knudsen M, Bohr VA, et al. Long-term artificial sweetener acesulfame potassium treatment alters neurometabolic functions in C57BL/6J mice. PLoS One. 2013;8:e70257.
Malaisse WJ, Vanonderbergen A, Louchami K, Jijakli H, Malaisse-Lagae F. Effects of artificial sweeteners on insulin release and cationic fluxes in rat pancreatic islets. Cell Signal. 1998;10:727–33.
Simon BR, Parlee SD, Learman BS, Mori H, Scheller EL, Cawthorn WP, et al. Artificial sweeteners stimulate adipogenesis and suppress lipolysis independently of sweet taste receptors. J Biol Chem. 2013;288:32475–89.
Zheng Y, Sarr MG. Effect of the artificial sweetener, acesulfame potassium, a sweet taste receptor agonist, on glucose uptake in small intestinal cell lines. J Gastrointest Surg. 2013;17:153–8 discussion p. 158.
Liang Y, Maier V, Steinbach G, Lalić L, Pfeiffer EF. The effect of artificial sweetener on insulin secretion. II. Stimulation of insulin release from isolated rat islets by Acesulfame K (in vitro experiments). Horm Metab Res. 1987;19:285–9.
Nakagawa Y, Nagasawa M, Yamada S, Hara A, Mogami H, Nikolaev VO, et al. Sweet taste receptor expressed in pancreatic β-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS One. 2009;4:e5106.
Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol. 2007;582:379–92.
Moran AW, Al-Rammahi MA, Arora DK, Batchelor DJ, Coulter EA, Daly K, et al. Expression of Na+/glucose co-transporter 1 (SGLT1) is enhanced by supplementation of the diet of weaning piglets with artificial sweeteners. Br J Nutr. 2010;104:637–46.
Smith K, Karimian-Azari E, LaMoia TE, Hussain T, Vargova V, Karolyi K, et al. T1R2 receptor-mediated glucose sensing in the upper intestine potentiates glucose absorption through activation of local regulatory pathways. Mol Metab. 2018.
Burke MV, Small DM. Physiological mechanisms by which non-nutritive sweeteners may impact body weight and metabolism. Physiol Behav. 2015;152:381–8.
Honka M-J, Latva-Rasku A, Bucci M, Virtanen KA, Hannukainen JC, Kalliokoski KK, et al. Insulin-stimulated glucose uptake in skeletal muscle, adipose tissue and liver: a positron emission tomography study. Eur J Endocrinol. 2018;178:523–31.
Goodpaster BH, Bertoldo A, Ng JM, Azuma K, Pencek RR, Kelley C, et al. Interactions among glucose delivery, transport, and phosphorylation that underlie skeletal muscle insulin resistance in obesity and type 2 Diabetes: studies with dynamic PET imaging. Diabetes. 2014;63:1058–68.
Swithers SE. Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocrinol Metab. 2013;24:431–41.
Swithers SE, Laboy AF, Clark K, Cooper S, Davidson TL. Experience with the high-intensity sweetener saccharin impairs glucose homeostasis and GLP-1 release in rats. Behav Brain Res. 2012;233:1–14.
Collison KS, Makhoul NJ, Zaidi MZ, Saleh SM, Andres B, Inglis A, et al. Gender dimorphism in aspartame-induced impairment of spatial cognition and insulin sensitivity. PLoS One. 2012;7:e31570.
Lammertsma AA. Forward to the Past: The Case for Quantitative PET Imaging. J Nucl Med. 2017;58:1019–24.
Malbert C-H, Picq C, Divoux J-L, Henry C, Horowitz M. Obesity-associated alterations in glucose metabolism are reversed by chronic bilateral stimulation of the abdominal vagus nerve. Diabetes. 2017;66:848–57.
Sylvetsky AC, Welsh JA, Brown RJ, Vos MB. Low-calorie sweetener consumption is increasing in the United States. Am J Clin Nutr. 2012;96:640–6.
Bahri S, Horowitz M, Malbert CH. Inward Glucose Transfer Accounts for Insulin-Dependent Increase in Brain Glucose Metabolism Associated with Diet-Induced Obesity. Obesity (Silver Spring). 2018.
Boellaard R. Standards for PET Image Acquisition and Quantitative Data Analysis. J Nucl Med. 2009;50:11S–20S.
Ilback N-G, Alzin M, Jahrl S, Enghardt-Barbieri H, Busk L. Estimated intake of the artificial sweeteners acesulfame-K, aspartame, cyclamate and saccharin in a group of Swedish diabetics. Food Addit Contam. 2003;20:115–26.
Val-Laillet D, Blat S, Louveau I, Malbert CH. A computed tomography scan application to evaluate adiposity in a minipig model of human obesity. Br J Nutr. 2010;104:1719–28.
Malbert C-H. AniMate-An open source software for absolute PET quantification. Annual Congress of the European Association of Nuclear Medicine. 2016:43.
Iozzo P, Gastaldelli A, Järvisalo MJ, Kiss J, Borra R, Buzzigoli E, et al. 18F-FDG assessment of glucose disposal and production rates during fasting and insulin stimulation: a validation study. J Nucl Med. 2006;47:1016–22.
Rehal MS, Fiskaare E, Tjäder I, Norberg Å, Rooyackers O, Wernerman J. Measuring energy expenditure in the intensive care unit: a comparison of indirect calorimetry by E-sCOVX and Quark RMR with Deltatrac II in mechanically ventilated critically ill patients. Crit Care. 2016;20:54.
Golay A, DeFronzo RA, Ferrannini E, Simonson DC, Thorin D, Acheson K, et al. Oxidative and non-oxidative glucose metabolism in non-obese type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1988;31:585–91.
Munk OL, Keiding S, Bass L. A method to estimate dispersion in sampling catheters and to calculate dispersion-free blood time-activity curves. Med Phys. 2008;35:3471–81.
Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–28.
Saikali S, Meurice P, Sauleau P, Eliat PA, Bellaud P, Randuineau G, et al. A three-dimensional digital segmented and deformable brain atlas of the domestic pig. J Neurosci Methods. 2010;192:102–9.
Poulsen PH, Smith DF, Ostergaard L, Danielsen EH, Gee A, Hansen SB, et al. In vivo estimation of cerebral blood flow, oxygen consumption and glucose metabolism in the pig by [15O]water injection, [15O]oxygen inhalation and dual injections of [18F]fluorodeoxyglucose. J Neurosci Methods. 1997;77:199–209.
Iozzo P, Jarvisalo MJ, Kiss J, Borra R, Naum GA, Viljanen A, et al. Quantification of liver glucose metabolism by positron emission tomography: validation study in pigs. Gastroenterology. 2007;132:531–42.
Honka H, Mäkinen J, Hannukainen JC, Tarkia M, Oikonen V, Teräs M, et al. Validation of [18F]fluorodeoxyglucose and positron emission tomography (PET) for the measurement of intestinal metabolism in pigs, and evidence of intestinal insulin resistance in patients with morbid obesity. Diabetologia. 2013;56:893–900.
Virtanen KA, Peltoniemi P, Marjamäki P, Asola M, Strindberg L, Parkkola R, et al. Human adipose tissue glucose uptake determined using [(18)F]-fluoro-deoxy-glucose ([(18)F]FDG) and PET in combination with microdialysis. Diabetologia. 2001;44:2171–9.
Peltoniemi P, Lönnroth P, Laine H, Oikonen V, Tolvanen T, Grönroos T, et al. Lumped constant for [(18)F]fluorodeoxyglucose in skeletal muscles of obese and nonobese humans. Am J Physiol Endocrinol Metab. 2000;279:E1122–30.
Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7.
Hong YT, Fryer TD. Kinetic modelling using basis functions derived from two-tissue compartmental models with a plasma input function: general principle and application to [18F]fluorodeoxyglucose positron emission tomography. Neuroimage. 2010;51:164–72.
Yakushev I, Drzezga A, Habeck C. Metabolic connectivity. Curr Opin Neurol. 2017;30:677–85.
Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8:e68910.
Hammers A, Allom R, Koepp MJ, Free SL, Myers R, Lemieux L, et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp. 2003;19:224–47.
Pepino MY. Metabolic effects of non-nutritive sweeteners. Physiol Behav. 2015;152:450–5.
Maersk M, Belza A, Stødkilde-Jørgensen H, Ringgaard S, Chabanova E, Thomsen H, et al. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr. 2012;95:283–9.
Mäkinen J, Hannukainen JC, Karmi A, Immonen HM, Soinio M, Nelimarkka L, et al. Obesity-associated intestinal insulin resistance is ameliorated after bariatric surgery. Diabetologia. 2015;58:1055–62.
Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–8.
Weygandt M, Mai K, Dommes E, Ritter K, Leupelt V, Spranger J, et al. Impulse control in the dorsolateral prefrontal cortex counteracts post-diet weight regain in obesity. Neuroimage. 2015;109:318–27.
Lee SH, Zabolotny JM, Huang H, Lee H, Kim YB. Insulin in the nervous system and the mind: Functions in metabolism, memory, and mood. Mol Metab. 2016;5:589–601.
Cheke LG, Bonnici HM, Clayton NS, Simons JS. Obesity and insulin resistance are associated with reduced activity in core memory regions of the brain. Neuropsychologia. 2017;96:137–49.
Tuulari JJ, Karlsson HK, Hirvonen J, Hannukainen JC, Bucci M, Helmiö M, et al. Weight loss after bariatric surgery reverses insulin-induced increases in brain glucose metabolism of the morbidly obese. Diabetes. 2013;62:2747–51.
Hirvonen J, Virtanen KA, Nummenmaa L, Hannukainen JC, Honka MJ, Bucci M, et al. Effects of insulin on brain glucose metabolism in impaired glucose tolerance. Diabetes. 2011;60:443–7.
Virtanen KA, Lönnroth P, Parkkola R, Peltoniemi P, Asola M, Viljanen T, et al. Glucose uptake and perfusion in subcutaneous and visceral adipose tissue during insulin stimulation in nonobese and obese humans. J Clin Endocrinol Metab. 2002;87:3902–10.
Iozzo P. Metabolic imaging in obesity: underlying mechanisms and consequences in the whole body. Ann N Y Acad Sci. 2015;1353:21–40.
Viner M, Mercier G, Hao F, Malladi A, Subramaniam RM. Liver SULmean at FDG PET/CT: interreader agreement and impact of placement of volume of interest. Radiology. 2013;267:596–601.
Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. European Association of Nuclear Medicine EANM: FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54.
Vállez Garcia D, Casteels C, Schwarz AJ, Dierckx RA, Koole M, Doorduin J. A standardized method for the construction of tracer specific PET and SPECT rat brain templates: validation and implementation of a toolbox. PLoS One. 2015;10:e0122363.
Acknowledgements
The authors thank staff of the UEPR unit for animal care, Mickael Genissel, Julien Georges, Alain Chauvin, Francis Le Gouevec, and Vincent Piedvache. We also thank Paula Aneb and Emilie Lebrun for their involvement in running the Aniscan imaging, and Raphael Comte (Pegase unit) for insulin measurements. The authors also thank Eric Bobillier for the development of the in-line radiation detector and robotic feeders.
Funding
The study was conducted within the Aniscan Imaging Center (Aniscan, INRA), which is supported by BPIFrance within the Investments for the Future Program.
Author information
Authors and Affiliations
Contributions
C-H.M. planned the experiments, conducted the studies, analyzed the data and wrote the manuscript. R.Y. and M.H. were involved in planning the experiments, writing the manuscript and interpretation of the data. C-H.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Disclosure of potential conflicts of interest
C-H. Malbert declares that he has no conflict of interest. M. Horowitz declares that he has no conflict of interest. R. Young declares that he has no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Preclinical Imaging
Electronic supplementary material
ESM 1
(DOCX 24 kb)
Rights and permissions
About this article
Cite this article
Malbert, CH., Horowitz, M. & Young, R.L. Low-calorie sweeteners augment tissue-specific insulin sensitivity in a large animal model of obesity. Eur J Nucl Med Mol Imaging 46, 2380–2391 (2019). https://doi.org/10.1007/s00259-019-04430-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04430-4