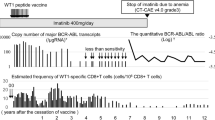
Abstract
Therapeutic cancer peptide vaccination is an immunotherapy designed to elicit cytotoxic T-lymphocyte (CTL) responses in patients. A number of therapeutic vaccination trials have been performed, nevertheless there are only a few reports that have analyzed the T-cell receptors (TCRs) expressed on tumor antigen-specific CTLs. Here, we use next-generation sequencing (NGS) to analyze TCRs of vaccine-induced CTL clones and the TCR repertoire of bulk T cells in peripheral blood mononuclear cells (PBMCs) from two lung cancer patients over the course of long-term vaccine therapy. In both patients, vaccination with two epitope peptides derived from cancer/testis antigens (upregulated lung cancer 10 (URLC10) and cell division associated 1 (CDCA1)) induced specific CTLs expressing various TCRs. All URLC10-specific CTL clones tested showed Ca2+ influx, IFN-γ production, and cytotoxicity when co-cultured with URLC10-pulsed tumor cells. Moreover, in CTL clones that were not stained with the URLC10/MHC-multimer, the CD3 ζ chain was not phosphorylated. NGS of the TCR repertoire of bulk PBMCs demonstrated that the frequency of vaccine peptide-specific CTL clones was near the minimum detectable threshold level. These results demonstrate that vaccination induces antigen-specific CTLs expressing various TCRs at different time points in cancer patients, and that some CTL clones are maintained in PBMCs during long-term treatment, including some with TCRs that do not bind peptide/MHC-multimer.

Similar content being viewed by others
Abbreviations
- CDCA1:
-
Cell division associated 1
- CDCA1-64:
-
CDCA1-derived HLA-A24 (A*24:02)-restricted peptide
- CDCA1/MHC-multimer:
-
CDCA1-64/ HLA-A*24:02 pentamer-PE
- CMV pp65 peptide:
-
CMV-derived HLA-A24 (A*24:02)-restricted peptide
- HIV epitope peptide:
-
HIV-derived HLA-A24 (A*24:02)-restricted peptide
- NGS:
-
Next-generation sequencing
- TC:
-
Treatment course
- URLC10:
-
Upregulated lung cancer 10
- URLC10-177:
-
URLC10-derived HLA-A24 (A*24:02)-restricted peptide
- URLC10/MHC-multimer:
-
URLC10-177/ HLA-A*24:02 tetramer-PE
- VEGFR:
-
Vascular endothelial growth factor receptor
References
Schlom J (2012) Therapeutic cancer vaccines: current status and moving forward. J Natl Cancer Inst 104(8):599–613. https://doi.org/10.1093/jnci/djs033
Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S, Thatcher N, Wagstaff J, Zielinski C, Faulkner I, Mellstedt H (2014) Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol 11(9):509–524. https://doi.org/10.1038/nrclinonc.2014.111
van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief CJ (2016) Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer 16(4):219–233. https://doi.org/10.1038/nrc.2016.16
Yadav M, Jhunjhunwala S, Phung QT, Lupardus P, Tanguay J, Bumbaca S, Franci C, Cheung TK, Fritsche J, Weinschenk T, Modrusan Z, Mellman I, Lill JR, Delamarre L (2014) Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature 515(7528):572–576. https://doi.org/10.1038/nature14001
Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, Mulder GE, Toebes M, Vesely MD, Lam SS, Korman AJ, Allison JP, Freeman GJ, Sharpe AH, Pearce EL, Schumacher TN, Aebersold R, Rammensee HG, Melief CJ, Mardis ER, Gillanders WE, Artyomov MN, Schreiber RD (2014) Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 515(7528):577–581. https://doi.org/10.1038/nature13988
Schumacher TN, Schreiber RD (2015) Neoantigens in cancer immunotherapy. Science 348(6230):69–74. https://doi.org/10.1126/science.aaa4971
Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, Ly A, Lie WR, Hildebrand WH, Mardis ER, Linette GP (2015) Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 348(6236):803–808. https://doi.org/10.1126/science.aaa3828
Yarchoan M, Johnson BA 3rd, Lutz ER, Laheru DA, Jaffee EM (2017) Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer 17(4):209–222. https://doi.org/10.1038/nrc.2016.154
Calis JJ, Rosenberg BR (2014) Characterizing immune repertoires by high throughput sequencing: strategies and applications. Trends Immunol 35(12):581–590. https://doi.org/10.1016/j.it.2014.09.004
Linnemann C, Heemskerk B, Kvistborg P, Kluin RJ, Bolotin DA, Chen X, Bresser K, Nieuwland M, Schotte R, Michels S, Gomez-Eerland R, Jahn L, Hombrink P, Legrand N, Shu CJ, Mamedov IZ, Velds A, Blank CU, Haanen JB, Turchaninova MA, Kerkhoven RM, Spits H, Hadrup SR, Heemskerk MH, Blankenstein T, Chudakov DM, Bendle GM, Schumacher TN (2013) High-throughput identification of antigen-specific TCRs by TCR gene capture. Nat Med 19(11):1534–1541. https://doi.org/10.1038/nm.3359
Kobayashi E, Mizukoshi E, Kishi H, Ozawa T, Hamana H, Nagai T, Nakagawa H, Jin A, Kaneko S, Muraguchi A (2013) A new cloning and expression system yields and validates TCRs from blood lymphocytes of patients with cancer within 10 days. Nat Med 19(11):1542–1546. https://doi.org/10.1038/nm.3358
Fang H, Yamaguchi R, Liu X, Daigo Y, Yew PY, Tanikawa C, Matsuda K, Imoto S, Miyano S, Nakamura Y (2014) Quantitative T cell repertoire analysis by deep cDNA sequencing of T cell receptor a and b chains using next-generation sequencing (NGS). Oncoimmunology 3(12):e968467. https://doi.org/10.4161/21624011.2014.968467
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515(7528):568–571. https://doi.org/10.1038/nature13954
Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, Herati RS, Mansfield KD, Patsch D, Amaravadi RK, Schuchter LM, Ishwaran H, Mick R, Pryma DA, Xu X, Feldman MD, Gangadhar TC, Hahn SM, Wherry EJ, Vonderheide RH, Minn AJ (2015) Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520(7547):373–377. https://doi.org/10.1038/nature14292
Inoue H, Park JH, Kiyotani K, Zewde M, Miyashita A, Jinnin M, Kiniwa Y, Okuyama R, Tanaka R, Fujisawa Y, Kato H, Morita A, Asai J, Katoh N, Yokota K, Akiyama M, Ihn H, Fukushima S, Nakamura Y (2016) Intratumoral expression levels of PD-L1, GZMA, and HLA-A along with oligoclonal T cell expansion associate with response to nivolumab in metastatic melanoma. Oncoimmunology 5(9):e1204507. https://doi.org/10.1080/2162402X.2016.1204507
Suzuki H, Fukuhara M, Yamaura T, Mutoh S, Okabe N, Yaginuma H, Hasegawa T, Yonechi A, Osugi J, Hoshino M, Kimura T, Higuchi M, Shio Y, Ise K, Takeda K, Gotoh M (2013) Multiple therapeutic peptide vaccines consisting of combined novel cancer testis antigens and anti-angiogenic peptides for patients with non-small cell lung cancer. J Transl Med 11:97. https://doi.org/10.1186/1479-5876-11-97
Yoshitake Y, Fukuma D, Yuno A, Hirayama M, Nakayama H, Tanaka T, Nagata M, Takamune Y, Kawahara K, Nakagawa Y, Yoshida R, Hirosue A, Ogi H, Hiraki A, Jono H, Hamada A, Yoshida K, Nishimura Y, Nakamura Y, Shinohara M (2015) Phase II clinical trial of multiple peptide vaccination for advanced head and neck cancer patients revealed induction of immune responses and improved OS. Clin Cancer Res 21(2):312–321. https://doi.org/10.1158/1078-0432.CCR-14-0202
Okuyama R, Aruga A, Hatori T, Takeda K, Yamamoto M (2013) Immunological responses to a multi-peptide vaccine targeting cancer-testis antigens and VEGFRs in advanced pancreatic cancer patients. Oncoimmunology 2(11):e27010. https://doi.org/10.4161/onci.27010
Kono K, Iinuma H, Akutsu Y, Tanaka H, Hayashi N, Uchikado Y, Noguchi T, Fujii H, Okinaka K, Fukushima R, Matsubara H, Ohira M, Baba H, Natsugoe S, Kitano S, Takeda K, Yoshida K, Tsunoda T, Nakamura Y (2012) Multicenter, phase II clinical trial of cancer vaccination for advanced esophageal cancer with three peptides derived from novel cancer-testis antigens. J Transl Med 10:141. https://doi.org/10.1186/1479-5876-10-141
Janetzki S, Panageas KS, Ben-Porat L, Boyer J, Britten CM, Clay TM, Kalos M, Maecker HT, Romero P, Yuan J, Kast WM, Hoos A, Elispot Proficiency Panel of the CVCIAWG (2008) Results and harmonization guidelines from two large-scale international Elispot proficiency panels conducted by the Cancer Vaccine Consortium (CVC/SVI). Cancer Immunol Immunother. 57(3): 303–315. https://doi.org/10.1007/s00262-007-0380-6
Suda T, Tsunoda T, Daigo Y, Nakamura Y, Tahara H (2007) Identification of human leukocyte antigen-A24-restricted epitope peptides derived from gene products upregulated in lung and esophageal cancers as novel targets for immunotherapy. Cancer Sci 98(11):1803–1808. https://doi.org/10.1111/j.1349-7006.2007.00603.x
Yoshimura S, Tsunoda T, Osawa R, Harada M, Watanabe T, Hikichi T, Katsuda M, Miyazawa M, Tani M, Iwahashi M, Takeda K, Katagiri T, Nakamura Y, Yamaue H (2014) Identification of an HLA-A2-restricted epitope peptide derived from hypoxia-inducible protein 2 (HIG2). PLoS One 9(1):e85267. https://doi.org/10.1371/journal.pone.0085267
Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, Kooistra T, Fingerle-Rowson G, Ghezzi P, Kleemann R, McColl SR, Bucala R, Hickey MJ, Weber C (2007) MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med 13(5):587–596. https://doi.org/10.1038/nm1567
Takeda K, Yamaguchi N, Akiba H, Kojima Y, Hayakawa Y, Tanner JE, Sayers TJ, Seki N, Okumura K, Yagita H, Smyth MJ (2004) Induction of tumor-specific T cell immunity by anti-DR5 antibody therapy. J Exp Med 199(4):437–448. https://doi.org/10.1084/jem.20031457
Kitaura K, Shini T, Matsutani T, Suzuki R (2016) A new high-throughput sequencing method for determining diversity and similarity of T cell receptor (TCR) a and b repertoires and identifying potential new invariant TCR a chains. BMC Immunol 17(1):38. https://doi.org/10.1186/s12865-016-0177-5
Gerlinger M, Quezada SA, Peggs KS, Furness AJ, Fisher R, Marafioti T, Shende VH, McGranahan N, Rowan AJ, Hazell S, Hamm D, Robins HS, Pickering L, Gore M, Nicol DL, Larkin J, Swanton C (2013) Ultra-deep T cell receptor sequencing reveals the complexity and intratumour heterogeneity of T cell clones in renal cell carcinomas. J Pathol 231(4):424–432. https://doi.org/10.1002/path.4284
Emerson RO, Sherwood AM, Rieder MJ, Guenthoer J, Williamson DW, Carlson CS, Drescher CW, Tewari M, Bielas JH, Robins HS (2013) High-throughput sequencing of T-cell receptors reveals a homogeneous repertoire of tumour-infiltrating lymphocytes in ovarian cancer. J Pathol 231(4):433–440. https://doi.org/10.1002/path.4260
Tamura K, Hazama S, Yamaguchi R, Imoto S, Takenouchi H, Inoue Y, Kanekiyo S, Shindo Y, Miyano S, Nakamura Y, Kiyotani K (2016) Characterization of the T cell repertoire by deep T cell receptor sequencing in tissues and blood from patients with advanced colorectal cancer. Oncol Lett 11(6):3643–3649. https://doi.org/10.3892/ol.2016.4465
Park JH, Jang M, Tarhan YE, Katagiri T, Sasa M, Miyoshi Y, Kalari KR, Suman VJ, Weinshilboum R, Wang L, Boughey JC, Goetz MP, Nakamura Y (2016) Clonal expansion of antitumor T cells in breast cancer correlates with response to neoadjuvant chemotherapy. Int J Oncol 49(2):471–478. https://doi.org/10.3892/ijo.2016.3540
Jang M, Yew PY, Hasegawa K, Ikeda Y, Fujiwara K, Fleming GF, Nakamura Y, Park JH (2015) Characterization of T cell repertoire of blood, tumor, and ascites in ovarian cancer patients using next generation sequencing. Oncoimmunology 4(11):e1030561. https://doi.org/10.1080/2162402X.2015.1030561
Subudhi SK, Aparicio A, Gao J, Zurita AJ, Araujo JC, Logothetis CJ, Tahir SA, Korivi BR, Slack RS, Vence L, Emerson RO, Yusko E, Vignali M, Robins HS, Sun J, Allison JP, Sharma P (2016) Clonal expansion of CD8 T cells in the systemic circulation precedes development of ipilimumab-induced toxicities. Proc Natl Acad Sci U S A 113(42):11919–11924. https://doi.org/10.1073/pnas.1611421113
Robert L, Tsoi J, Wang X, Emerson R, Homet B, Chodon T, Mok S, Huang RR, Cochran AJ, Comin-Anduix B, Koya RC, Graeber TG, Robins H, Ribas A (2014) CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clin Cancer Res 20(9):2424–2432. https://doi.org/10.1158/1078-0432.CCR-13-2648
Leisegang M, Engels B, Schreiber K, Yew PY, Kiyotani K, Idel C, Arina A, Duraiswamy J, Weichselbaum RR, Uckert W, Nakamura Y, Schreiber H (2016) Eradication of large solid tumors by gene therapy with a T-cell receptor targeting a single cancer-specific point mutation. Clin Cancer Res 22(11):2734–2743. https://doi.org/10.1158/1078-0432.CCR-15-2361
Engels B, Engelhard VH, Sidney J, Sette A, Binder DC, Liu RB, Kranz DM, Meredith SC, Rowley DA, Schreiber H (2013) Relapse or eradication of cancer is predicted by peptide-major histocompatibility complex affinity. Cancer Cell 23(4):516–526. https://doi.org/10.1016/j.ccr.2013.03.018
Hernández J, Lee PP, Davis MM, Sherman LA (2000) The use of HLA A2.1/p53 peptide tetramers to visualize the impact of self tolerance on the TCR repertoire. J Immunol 164(2):596–602
Burrows SR, Kienzle N, Winterhalter A, Bharadwaj M, Altman JD, Brooks A (2000) Peptide-MHC class I tetrameric complexes display exquisite ligand specificity. J Immunol 165(11):6229–6234
Buslepp J, Zhao R, Donnini D, Loftus D, Saad M, Appella E, Collins EJ (2001) T cell activity correlates with oligomeric peptide-major histocompatibility complex binding on T cell surface. J Biol Chem 276(50):47320–47328. https://doi.org/10.1074/jbc.M109231200
Laugel B, van den Berg HA, Gostick E, Cole DK, Wooldridge L, Boulter J, Milicic A, Price DA, Sewell AK (2007) Different T cell receptor affinity thresholds and CD8 coreceptor dependence govern cytotoxic T lymphocyte activation and tetramer binding properties. J Biol Chem 282(33):23799–23810. https://doi.org/10.1074/jbc.M700976200
Stone JD, Artyomov MN, Chervin AS, Chakraborty AK, Eisen HN, Kranz DM (2011) Interaction of streptavidin-based peptide-MHC oligomers (tetramers) with cell-surface TCRs. J Immunol 187(12):6281–6290. https://doi.org/10.4049/jimmunol.1101734
Sabatino JJ Jr, Huang J, Zhu C, Evavold BD (2011) High prevalence of low affinity peptide-MHC II tetramer-negative effectors during polyclonal CD4+ T cell responses. J Exp Med 208(1):81–90. https://doi.org/10.1084/jem.20101574
Schubert DA, Gordo S, Sabatino JJ Jr, Vardhana S, Gagnon E, Sethi DK, Seth NP, Choudhuri K, Reijonen H, Nepom GT, Evavold BD, Dustin ML, Wucherpfennig KW (2012) Self-reactive human CD4 T cell clones form unusual immunological synapses. J Exp Med 209(2):335–352. https://doi.org/10.1084/jem.20111485
Sharma P, Allison JP (2015) The future of immune checkpoint therapy. Science 348(6230):56–61. https://doi.org/10.1126/science.aaa8172
Kamphorst AO, Wieland A, Nasti T, Yang S, Zhang R, Barber DL, Konieczny BT, Daugherty CZ, Koenig L, Yu K, Sica GL, Sharpe AH, Freeman GJ, Blazar BR, Turka LA, Owonikoko TK, Pillai RN, Ramalingam SS, Araki K, Ahmed R (2017) Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science 355(6332):1423–1427. https://doi.org/10.1126/science.aaf0683
Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, Sasmal DK, Huang J, Kim JM, Mellman I, Vale RD (2017) T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 355(6332):1428–1433. https://doi.org/10.1126/science.aaf1292
Takeda K, Kojima Y, Uno T, Hayakawa Y, Teng MW, Yoshizawa H, Yagita H, Gejyo F, Okumura K, Smyth MJ (2010) Combination therapy of established tumors by antibodies targeting immune activating and suppressing molecules. J Immunol 184(10):5493–5501. https://doi.org/10.4049/jimmunol.0903033
Acknowledgements
We are grateful to OncoTherapy Science, Inc. (Kanagawa, Japan) for their technical support.
Funding
This work was supported by the Ministry of Education, Science, and Culture, Japan (15K14410) to K. Takeda.
Author information
Authors and Affiliations
Contributions
Kazuyoshi Takeda designed this study, interpreted the data, and wrote the manuscript. Kazutaka Kitaura and Ryuji Suzuki carried out next-generation sequencing. Yuki Owada, Satoshi Muto, Naoyuki Okabe, Takeo Hasegawa, Jun Osugi, and Mika Hoshino carried out biological analysis. Takuya Tsunoda, Ko Okumura and Hiroyuki Suzuki revised the manuscript. All authors had final approval of the submitted and published versions.
Corresponding author
Ethics declarations
Conflict of interest
K. Kitaura and R. Suzuki are currently employed by Repertoire Genesis, Inc. The other authors declare that they have no conflicts of interest.
Ethical approval and ethical standards
This study was approved by the ethical committee of Fukushima Medical University (approval number: 810) and was registered with ClinicalTrials.gov (NCT00874588). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any animal studies performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Takeda, K., Kitaura, K., Suzuki, R. et al. Quantitative T-cell repertoire analysis of peripheral blood mononuclear cells from lung cancer patients following long-term cancer peptide vaccination. Cancer Immunol Immunother 67, 949–964 (2018). https://doi.org/10.1007/s00262-018-2152-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-018-2152-x