Abstract
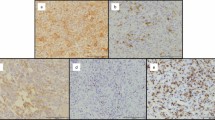
Giant cell tumor of bone (GCTB) is a locally aggressive and rarely metastatic tumor, with a relatively unpredictable clinical course. A retrospective series of 46 GCTB and a control group of 24 aneurysmal bone cysts (ABC) were selected with the aim of investigating the PD-L1 expression levels and immune-related gene expression profile, in correlation with clinicopathological features. PD-L1 and Ki67 were immunohistochemically tested in each case. Furthermore, comprehensive molecular analyses were carried out using NanoString technology and nCounter PanCancer Immune Profiling Panel, and the gene expression results were correlated with clinicopathological characteristics. PD-L1 expression was observed in 13/46 (28.3%) GCTB (and in 1/24, 4.2%, control ABC, only) and associated with a shorter disease free interval according to univariate analysis. Moreover, in PD-L1-positive lesions, three genes (CD27, CD6 and IL10) were significantly upregulated (p < 0.01), while two were downregulated (LCK and TLR8, showing borderline significance, p = 0.06). Interestingly, these genes can be related to maturation and immune tolerance of bone tissue microenvironment, suggesting a more immature/anergic phenotype of giant cell tumors. Our findings suggest that PD-L1 immunoreactivity may help to select GCTB patients with a higher risk of recurrence who could potentially benefit from immune checkpoint blockade.



Similar content being viewed by others
Abbreviations
- ABC:
-
Aneurysmal bone cyst
- DFI:
-
Disease-free interval
- FFPE:
-
Formalin-fixed paraffin embedded
- GC:
-
Giant cell
- GCTB:
-
Giant cell tumor of bone
- H&E:
-
Hematoxylin and eosin
- IHC:
-
Immunohistochemistry
- MTC:
-
Mononuclear tumor cell
- SD:
-
Standard deviation
- WHO:
-
World Health Organization
REFERENCES
Cowan RW, Singh G (2013) Giant cell tumor of bone: a basic science perspective. Bone 52:238–246. https://doi.org/10.1016/j.bone.2012.10.002
Athanasou NA, Bansal M, Forsyth R, Reid RP, Sapi Z (2013) Giant cell tumour of bone. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F (eds) WHO classification of tumours of soft tissue and bone, 4th edn. IARC Press, Lyon, pp 321–324
Errani C, Ruggieri P, Asenzio MA, Toscano A, Colangeli S, Rimondi E et al (2010) Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat Rev 36:1–7. https://doi.org/10.1016/j.ctrv.2009.09.002
Werner M (2006) Giant cell tumour of bone: morphological, biological and histogenetical aspects. Int Orthop 30:484–489. https://doi.org/10.1007/s00264-006-0215-7
Luo G, Li F, Li X, Wang ZG, Zhang B (2018) TNF-α and RANKL promote osteoclastogenesis by upregulating RANK via the NF-κB pathway. Mol Med Rep 17:6605–6611. https://doi.org/10.3892/mmr.2018.8698
Atkins GJ, Haynes DR, Graves SE, Evdokiou A, Hay S, Bouralexis S, Findlay DM (2000) Expression of osteoclast differentiation signals by stromal elements of giant cell tumors. J Bone Miner Res 15:640–649. https://doi.org/10.1359/jbmr.2000.15.4.640
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423:337–342. https://doi.org/10.1038/nature01658
Maros ME, Schnaidt S, Balla P, Kelemen Z, Sapi Z et al (2019) In situ cell cycle analysis in giant cell tumor of bone reveals patients with elevated risk of reduced progression-free survival. Bone 127:188–198. https://doi.org/10.1016/j.bone.2019.06.022
Kervarrec T, Collin C, Larousserie F, Bouvier C, Aubert S, Gomez-Brouchet A et al (2017) H3F3 mutation status of giant cell tumors of the bone, chondroblastomas and their mimics: a combined high resolution melting and pyrosequencing approach. Mod Pathol 30:393–406. https://doi.org/10.1038/modpathol.2016.212
Righi A, Mancini I, Gambarotti M, Picci P, Gamberi G, Marraccini C et al (2017) Histone 3.3 mutations in giant cell tumor and giant cell-rich sarcomas of bone. Hum Pathol 68:128–135. https://doi.org/10.1016/j.humpath.2017.08.033
Lüke J, von Baer A, Schreiber J, Lübbehüsen C, Breining T, Mellert K et al (2017) H3F3A mutation in giant cell tumour of the bone is detected by immunohistochemistry using a monoclonal antibody against the G34W mutated site of the histone H3.3 variant. Histopathology 71:125–133. https://doi.org/10.1111/his.13190
Presneau N, Baumhoer D, Behjati S, Pillay N, Tarpey P, Campbell PJ et al (2015) Diagnostic value of H3F3A mutations in giant cell tumour of bone compared to osteoclast-rich mimics. J Pathol Clin Res 1:113–123. https://doi.org/10.1002/cjp2.13
Montgomery C, Couch C, Emory CL, Nicholas R (2019) Giant cell tumor of bone: review of current literature, evaluation, and treatment options. J Knee Surg 32:331–336. https://doi.org/10.1055/s-0038-1675815
Chen L, Ding XY, Wang CS, Si MJ, Du LJ, Zhang WB, Lu Y (2014) In-depth analysis of local recurrence of giant cell tumour of bone with soft tissue extension after intralesional curettage. Radiol Med 119:861–870. https://doi.org/10.1007/s11547-014-0396-x
Errani C, Tsukamoto S, Leone G, Akahane M, Cevolani L, Tanzi P et al (2017) Higher local recurrence rates after intralesional surgery for giant cell tumor of the proximal femur compared to other sites. Eur J Orthop Surg Traumatol 27:813–819. https://doi.org/10.2106/JBJS.17.00057
Lausten GS, Jensen PK, Schiødt T, Lund B (1996) Local recurrences in giant cell tumour of bone. Long-term follow up of 31 cases. Int Orthop 20:172–176. https://doi.org/10.1007/s002640050057
Branstetter DG, Nelson SD, Manivel JC, Blay JY, Chawla S, Thomas DM et al (2012) Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin Cancer Res 18:4415–4424. https://doi.org/10.1158/1078-0432.CCR-12-0578
Luengo-Alonso G, Mellado-Romero M, Shemesh S, Ramos-Pascua L, Pretell-Mazzini J (2019) Denosumab treatment for giant-cell tumor of bone: a systematic review of the literature. Arch Orthop Trauma Surg. https://doi.org/10.1007/s00402-019-03167-x
Chawla S, Henshaw R, Seeger L, Choy E, Blay JY, Ferrari S et al (2013) Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol 14:901–908. https://doi.org/10.1016/S1470-2045(13)70277-8
Errani C, Tsukamoto S, Leone G, Righi A, Akahane M, Tanaka Y, Donati DM (2018) Denosumab may increase the risk of local recurrence in patients with giant-cell tumor of bone treated with curettage. J Bone Joint Surg Am 100:496–504. https://doi.org/10.2106/JBJS.17.00057
van der Heijden L, Dijkstra PDS, Blay JY, Gelderblom H (2017) Giant cell tumour of bone in the denosumab era. Eur J Cancer 77:75–83. https://doi.org/10.1016/j.ejca.2017.02.021
Errani C, Tsukamoto S, Mavrogenis AF (2017) How safe and effective is denosumab for bone giant cell tumour? Int Orthop 41:2397–2400. https://doi.org/10.1007/s00264-017-3536-9
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Bone Cancer. https://www.nccn.org/professionals/physician_gls/default.aspx#site Accessed 26 June 2019
Domingues B, Lopes JM, Soares P, Pópulo H (2018) Melanoma treatment in review. Immunotargets Ther 7:35–49. https://doi.org/10.2147/ITT.S134842
Yu Y, Cui J (2018) Present and future of cancer immunotherapy: a tumor microenvironmental perspective. Oncol Lett 16:4105–4113. https://doi.org/10.3892/ol.2018.9219
Wang Z, Wang Z, Li B, Wang S, Chen T, Ye Z (2019) Innate immune cells: a potential and promising cell population for treating osteosarcoma. Front Immunol 10:1114. https://doi.org/10.3389/fimmu.2019.01114
Uehara T, Fujiwara T, Takeda K, Kunisada T, Ozaki T, Udono H (2015) Immunotherapy for bone and soft tissue sarcomas. Biomed Res Int 2015:820813. https://doi.org/10.1155/2015/820813
Zheng W, Xiao H, Liu H, Zhou Y (2015) Expression of programmed death 1 is correlated with progression of osteosarcoma. APMIS 123:102–107. https://doi.org/10.1111/apm.12311
Torabi A, Amaya CN, Wians FH Jr, Bryan BA (2017) PD-1 and PD-L1 expression in bone and soft tissue sarcomas. Pathology 49:506–513. https://doi.org/10.1016/j.pathol.2017.05.003
McEachron TA, Triche TJ, Sorenson L, Parham DM, Carpten JD (2018) Profiling targetable immune checkpoints in osteosarcoma. Oncoimmunology 7:e1475873. https://doi.org/10.1080/2162402X.2018.1475873
Wang J, Hu C, Wang J, Shen Y, Bao Q, He F et al (2019) Checkpoint blockade in combination with doxorubicin augments tumor cell apoptosis in osteosarcoma. J Immunother. https://doi.org/10.1097/CJI.0000000000000281
Rehkämper J, Steinestel K, Jeiler B, Elges S, Hekeler E, Huss S et al (2018) Diagnostic tools in the differential diagnosis of giant cell-rich lesions of bone at biopsy. Oncotarget 9:30106–30114. https://doi.org/10.18632/oncotarget.25725
Urakawa H, Yonemoto T, Matsumoto S, Takagi T, Asanuma K, Watanuki M et al (2018) Clinical outcome of primary giant cell tumor of bone after curettage with or without perioperative denosumab in Japan: from a questionnaire for JCOG 1610 study. World J Surg Oncol 16:160. https://doi.org/10.1186/s12957-018-1459-6
Yoshida K, Okamoto M, Sasaki J, Kuroda C, Ishida H, Ueda K et al (2019) Clinical outcome of osteosarcoma and its correlation with programmed death-ligand 1 and T cell activation markers. Onco Targets Ther 12:2513–2518. https://doi.org/10.2147/OTT.S198421
Kim JR, Moon YJ, Kwon KS, Bae JS, Wagle S, Kim KM et al (2013) Tumor infiltrating PD1-positive lymphocytes and the expression of PD-L1 predict poor prognosis of soft tissue sarcomas. PLoS One 8:e82870. https://doi.org/10.1371/journal.pone.0082870
Kim C, Kim EK, Jung H, Chon HJ, Han JW, Shin KH et al (2016) Prognostic implications of PD-L1 expression in patients with soft tissue sarcoma. BMC Cancer 16:434. https://doi.org/10.1186/s12885-016-2451-6
Xiao Y, Song JY, de Vries TJ, Fatmawati C, Parreira DB, Langenbach GE et al (2013) Osteoclast precursors in murine bone marrow express CD27 and are impeded in osteoclast development by CD70 on activated immune cells. Proc Natl Acad Sci USA 110:12385–12390. https://doi.org/10.1073/pnas.1216082110
Bozec A, Zaiss MM (2017) T regulatory cells in bone remodelling. Curr Osteoporos Rep 15:121–125. https://doi.org/10.1007/s11914-017-0356-1
Oliveira MI, Gonçalves CM, Pinto M, Fabre S, Santos AM, Lee SF et al (2012) CD6 attenuates early and late signaling events, setting thresholds for T-cell activation. Eur J Immunol 42:195–205. https://doi.org/10.1002/eji.201040528
Kato I, Furuya M, Matsuo K, Kawabata Y, Tanaka R, Ohashi K (2018) Giant cell tumours of bone treated with denosumab: histological, immunohistochemical and H3F3A mutation analyses. Histopathology 72:914–922. https://doi.org/10.1111/his.13448
Solari F, Domenget C, Gire V, Woods C, Lazarides E, Rousset B, Jurdic P (1995) Multinucleated cells can continuously generate mononucleated cells in the absence of mitosis: a study of cells of the avian osteoclast lineage. J Cell Sci 108:3233–3241
Niu N, Zhang J, Zhang N, Mercado-Uribe I, Tao F, Han Z et al (2016) Linking genomic reorganization to tumor initiation via the giant cell cycle. Oncogenesis 5:e281. https://doi.org/10.1038/oncsis.2016.75
Janssens S, Beyaert R (2003) Role of Toll-like receptors in pathogen recognition. Clin Microbiol Rev 16:637–646. https://doi.org/10.1128/CMR.16.4.637-646.2003
Schmiedel BJ, Grosse-Hovest L, Salih HR (2013) A “vicious cycle” of NK-cell immune evasion in acute myeloid leukemia mediated by RANKL? Oncoimmunology 2:e23850. https://doi.org/10.4161/onci.23850
Gorski KS, Waller EL, Bjornton-Severson J, Hanten JA, Riter CL, Kieper WC et al (2006) Distinct indirect pathways govern human NK-cell activation by TLR-7 and TLR-8 agonists. Int Immunol 18:1115–1126. https://doi.org/10.1093/intimm/dxl046
Huang L, Zhu P, Xia P, Fan Z (2016) WASH has a critical role in NK cell cytotoxicity through Lck-mediated phosphorylation. Cell Death Dis 7:e2301. https://doi.org/10.1038/cddis.2016.212
Francisconi CF, Vieira AE, Azevedo MCS, Tabanez AP, Fonseca AC, Trombone APF et al (2018) RANKL triggers treg-mediated immunoregulation in inflammatory osteolysis. J Dent Res 97:917–927. https://doi.org/10.1177/0022034518759302
Palmerini E, Chawla NS, Ferrari S, Sudan M, Picci P, Marchesi E et al (2017) Denosumab in advanced/unresectable giant-cell tumour of bone (GCTB): for how long? Eur J Cancer 76:118–124. https://doi.org/10.1016/j.ejca.2017.01.028
Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR et al (2017) IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 127:2930–2940. https://doi.org/10.1172/JCI91190
Danaher P, Warren S, Lu R, Samayoa J, Sullivan A, Pekker I et al (2018) Pan-cancer adaptive immune resistance as defined by the Tumor Inflammation Signature (TIS): results from The Cancer Genome Atlas (TCGA). J Immunother Cancer 6:63. https://doi.org/10.1186/s40425-018-0367-1
Klein S, Mauch C, Wagener-Ryczek S, Schoemmel M, Buettner R, Quaas A et al (2019) Immune-phenotyping of pleomorphic dermal sarcomas suggests this entity as a potential candidate for immunotherapy. Cancer Immunol Immunother 68:973–982. https://doi.org/10.1007/s00262-019-02339-3
Acknowledgments
We would like to thank Mr. Lorenzo Visca for his skillful technical assistance. This study is dedicated to the memory of Tom, an extraordinarily lively boy who untimely died of cancer.
Funding
This work was partially supported by an unrestricted grant (1704/2018) from the “Fondazione per i Tumori muscolo scheletrici,” Torino. In addition, this research received funding specifically dedicated to the Department of Medical Sciences from Italian Ministry for Education, University and Research (Ministero dell’Istruzione, dell’Università e della Ricerca—MIUR) under the program “Dipartimenti di Eccellenza 2018–2022,″ Project no D15D18000410001. JM and LB are PhD fellows at the University of Turin. LA is funded by Fondazione Umberto Veronesi (Postdoctoral fellowship 2018 and 2019).
Author information
Authors and Affiliations
Contributions
MP designed and supervised the study. JM and AL collected the data and prepared the material. LA, JM, CM, FV and CV analyzed the samples. AC, GG, RP and NR treated and followed patients and provided clinical data. SOA performed statistical analyses. MP, JM, LA, SOA, AL, LB and PC contributed to data interpretation. The first draft of the manuscript was written by MP and JM, and all authors critically revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical approval and ethical standards
This study was conducted in accordance with the ethical standards of the Declaration of Helsinki, and approval was granted by the Research Ethics Committee for Human Biospecimen Utilization (Department of Medical Sciences—ChBU) of the University of Turin (n° 10/2019). This study does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Before the study started, all cases were de-identified and coded by a pathology staff member not involved in the study, and all data were accessed anonymously. Considering the retrospective nature of this research protocol with no impact on patients’ treatment and the use of anonymized data only, no written consent was required by the Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Metovic, J., Annaratone, L., Linari, A. et al. Prognostic role of PD-L1 and immune-related gene expression profiles in giant cell tumors of bone. Cancer Immunol Immunother 69, 1905–1916 (2020). https://doi.org/10.1007/s00262-020-02594-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02594-9