Abstract
Background
Chemotherapy for non-small cell lung cancer (NSCLC) patients with preexisting interstitial lung diseases (ILDs) increases the risk of developing pneumonitis. However, the association between pneumonitis and immune checkpoint inhibitors (ICIs) and related factors remains unclear.
Methods
We conducted a retrospective cohort study using a nationwide inpatient database. We included patients (aged ≥ 20 years) newly diagnosed with ILD and NSCLC and who started chemotherapy (ICIs or conventional chemotherapy) between January 2016 and December 2019. The primary endpoint was the onset of pneumonitis. We estimated the cumulative incidence function of pneumonitis and compared it with patients taking ICIs and patients receiving conventional chemotherapy using Gray’s test. We calculated the subdistribution hazard ratios (HRs) and 95% confidence intervals (CIs) for the incidence of pneumonitis using Fine and Gray’s model to adjust for sex, age, smoking status, histology of NSCLC, surgical history, and medical histories, considering death as the competing risk.
Results
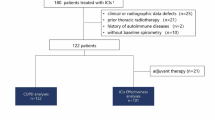
We identified 1177 patients (mean age 72 years, 13.8% female), of which 328 and 849 were in the ICI and conventional chemotherapy groups, respectively. There was no significant difference in the cumulative incidence function of pneumonitis between the two groups (p = 0.868). The adjusted subdistribution HR for the incidence of pneumonitis was 1.08 (95% CI: 0.74–1.57). Age (≥ 65 years) (HR: 1.86, 95% CI: 1.11–3.10) and smoking history (HR: 2.04, 95% CI: 1.02–4.11) were associated with developing pneumonitis.
Conclusion
The risk of developing pneumonitis with ICIs for NSCLC patients with preexisting ILD was similar to that with conventional chemotherapy.



Similar content being viewed by others
References
Omori T, Tajiri M, Baba T, Ogura T, Iwasawa T, Okudela K et al (2015) Pulmonary resection for lung cancer in patients with idiopathic interstitial pneumonia. Ann Thorac Surg 100(3):954–960. https://doi.org/10.1016/j.athoracsur.2015.03.094
Sato T, Teramukai S, Kondo H, Watanabe A, Ebina M, Kishi K et al (2014) Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg 147(5):1604–11 e3. https://doi.org/10.1016/j.jtcvs.2013.09.050
Kenmotsu H, Naito T, Kimura M, Ono A, Shukuya T, Nakamura Y et al (2011) The risk of cytotoxic chemotherapy-related exacerbation of interstitial lung disease with lung cancer. J Thorac Oncol 6(7):1242–1246. https://doi.org/10.1097/JTO.0b013e318216ee6b
Kenmotsu H, Naito T, Mori K, Ko R, Ono A, Wakuda K et al (2015) Effect of platinum-based chemotherapy for non-small cell lung cancer patients with interstitial lung disease. Cancer Chemother Pharmacol 75(3):521–526. https://doi.org/10.1007/s00280-014-2670-y
Minegishi Y, Sudoh J, Kuribayasi H, Mizutani H, Seike M, Azuma A et al (2011) The safety and efficacy of weekly paclitaxel in combination with carboplatin for advanced non-small cell lung cancer with idiopathic interstitial pneumonias. Lung Cancer 71(1):70–74. https://doi.org/10.1016/j.lungcan.2010.04.014
Watanabe N, Taniguchi H, Kondoh Y, Kimura T, Kataoka K, Nishiyama O et al (2014) Chemotherapy for extensive-stage small-cell lung cancer with idiopathic pulmonary fibrosis. Int J Clin Oncol 19(2):260–265. https://doi.org/10.1007/s10147-013-0554-5
Suresh K, Naidoo J, Lin CT, Danoff S (2018) Immune checkpoint immunotherapy for non-small cell lung cancer: benefits and pulmonary toxicities. Chest 154(6):1416–1423. https://doi.org/10.1016/j.chest.2018.08.1048
Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F et al (2018) Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 4(12):1721–1728. https://doi.org/10.1001/jamaoncol.2018.3923
Shibaki R, Murakami S, Matsumoto Y, Yoshida T, Goto Y, Kanda S et al (2020) Association of immune-related pneumonitis with the presence of preexisting interstitial lung disease in patients with non-small lung cancer receiving anti-programmed cell death 1 antibody. Cancer Immunol Immunother 69(1):15–22. https://doi.org/10.1007/s00262-019-02431-8
Isono T, Kagiyama N, Takano K, Hosoda C, Nishida T, Kawate E et al (2021) Outcome and risk factor of immune-related adverse events and pneumonitis in patients with advanced or postoperative recurrent non-small cell lung cancer treated with immune checkpoint inhibitors. Thorac Cancer 12(2):153–164. https://doi.org/10.1111/1759-7714.13736
Kudoh S, Kato H, Nishiwaki Y, Fukuoka M, Nakata K, Ichinose Y et al (2008) Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am J Respir Crit Care Med 177(12):1348–1357. https://doi.org/10.1164/rccm.200710-1501OC
Cui P, Liu Z, Wang G, Ma J, Qian Y, Zhang F et al (2018) Risk factors for pneumonitis in patients treated with anti-programmed death-1 therapy: a case-control study. Cancer Med 7(8):4115–4120. https://doi.org/10.1002/cam4.1579
Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J et al (2017) Pneumonitis in patients treated with anti-programmed death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol 35(7):709–717. https://doi.org/10.1200/JCO.2016.68.2005
Huang Y, Fan H, Li N, Du J (2019) Risk of immune-related pneumonitis for PD1/PD-L1 inhibitors: systematic review and network meta-analysis. Cancer Med 8(5):2664–2674. https://doi.org/10.1002/cam4.2104
Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J et al (2021) First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol 22(2):198–211. https://doi.org/10.1016/S1470-2045(20)30641-0
Nakashima M, Takeuchi M, Kawakami K (2020) Effectiveness and safety of Regorafenib vs. Trifluridine/Tipiracil in unresectable colorectal cancer: a retrospective cohort study. Clin Colorectal Cancer 19(4):e208–e25. https://doi.org/10.1016/j.clcc.2020.05.003
Yamada S, Sato I, Kawakami K (2019) A descriptive epidemiological study on the treatment options for head and neck cancer: transition before and after approval of cetuximab. Pharmacoepidemiol Drug Saf 28(3):330–336. https://doi.org/10.1002/pds.4703
Medical.data.Vision Co., Ltd. Press release. [Available from: https://www.mdv.co.jp/press/2020/detail_1471.html.
Ishii M (2012) DRG/PPS and DPC/PDPS as prospective payment systems. Japan Med Assoc J 55(4):279–291
Tanaka S, Seto K, Kawakami K (2015) Pharmacoepidemiology in Japan: medical databases and research achievements. J Pharm Health Care Sci 1:16. https://doi.org/10.1186/s40780-015-0016-5
Charlson M, Szatrowski TP, Peterson J, Gold J (1994) Validation of a combined comorbidity index. J Clin Epidemiol 47(11):1245–1251. https://doi.org/10.1016/0895-4356(94)90129-5
Cho JY, Kim J, Lee JS, Kim YJ, Kim SH, Lee YJ et al (2018) Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung Cancer 125:150–156. https://doi.org/10.1016/j.lungcan.2018.09.015
Yamaguchi T, Shimizu J, Hasegawa T, Horio Y, Inaba Y, Yatabe Y et al (2018) Pre-existing pulmonary fibrosis is a risk factor for anti-PD-1-related pneumonitis in patients with non-small cell lung cancer: a retrospective analysis. Lung Cancer 125:212–217. https://doi.org/10.1016/j.lungcan.2018.10.001
Maillet D, Corbaux P, Stelmes JJ, Dalle S, Locatelli-Sanchez M, Perier-Muzet M et al (2020) Association between immune-related adverse events and long-term survival outcomes in patients treated with immune checkpoint inhibitors. Eur J Cancer 132:61–70. https://doi.org/10.1016/j.ejca.2020.03.017
Navaratnam V, Fogarty AW, Glendening R, McKeever T, Hubbard RB (2013) The increasing secondary care burden of idiopathic pulmonary fibrosis: hospital admission trends in England from 1998 to 2010. Chest 143(4):1078–1084. https://doi.org/10.1378/chest.12-0803
Postow MA, Sidlow R, Hellmann MD (2018) Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378(2):158–168. https://doi.org/10.1056/NEJMra1703481
Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM et al (2018) Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 36(17):1714–1768. https://doi.org/10.1200/JCO.2017.77.6385
Acknowledgements
We would like to thank Editage (http://www.editage.com) for English-language editing of this manuscript.
Author information
Authors and Affiliations
Contributions
KS designed the study, performed the analysis (and took responsibility for data integrity and accuracy), discussed the results, and drafted the manuscript. IS designed the study, performed the analysis, discussed the results, and drafted the manuscript. MT designed the study, discussed the results, and drafted the manuscript. KK directed the project, obtained funding and accessible source data, designed the study, discussed the results, and drafted the manuscript. All authors contributed to revisions of the manuscript for critically important content and approved this version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
K. Sawa receives payment for lectures from Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., and Nippon Boehringer Ingelheim Co., Ltd. I. Sato holds stack in Astellas Pharma Inc. and Daiichi Sankyo Co., Ltd.M. Takeuchi received a consultation fee from Eisai Co., Ltd. K. Kawakami received advisory fees from Shin Nippon Biomedical Laboratories, Ltd., JMDC Inc., Leber Inc. Kaken Pharmaceutical Co.,Ltd., and Advanced Medical Care Inc.; payment for lectures including service on speakers bureaus from Mitsubishi Corp., Mitsubishi Chemical Holdings Corp., Daiichi Sankyo Co., Ltd., Chugai Pharmaceutical Co., Ltd., Kyoto University Original Co., Ltd., McKinsey & Co. Inc., Nikkei Business Publications, Inc., Astellas Pharma Inc., AstraZeneca K.K., Mitsubishi Tanabe Pharma Corp., and IQVIA Services Japan K.K.; research funds from Sumitomo Dainippon Pharma Co., Ltd., Pfizer Inc., Stella Pharma Corporation, CMIC Co., Ltd., Suntory Beverage & Food Ltd., Eisai Co., Ltd., Kyowa Kirin Co., Ltd., Mitsubishi Corp. Ltd. and Real World Data, Co., Ltd.; and holds stock in Real World Data, Co., Ltd.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sawa, K., Sato, I., Takeuchi, M. et al. Risk of pneumonitis in non-small cell lung cancer patients with preexisting interstitial lung diseases treated with immune checkpoint inhibitors: a nationwide retrospective cohort study. Cancer Immunol Immunother 72, 591–598 (2023). https://doi.org/10.1007/s00262-022-03281-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03281-7