Abstract
Purpose
The aims of the present study were: (1) to characterize the bone-marrow aspirate (BMA) obtained with a centrifuge-free process, employing a dedicated aspiration device; (2) to test the in vitro efficacy of BMA in a model of cartilage inflammation; and (3) to report the preliminary clinical results in a small cohort of patients affected by knee OA.
Methods
Ten patients (4 M, 6 W; mean age: 51.9 ± 9.2 yy) affected by mild to moderate unicompartmental knee OA (KL grade 2–3) were treated by intra-articular and subchondral injections of BMA obtained by a centrifuge-free process. To evaluate the effectiveness of the device in harvesting mesenchymal stem cells (MSCs), samples of the obtained BMA were tested by flow cytometry before and after subculture; BMA ability to counteract inflammation was also tested in an in vitro model of cartilage cell inflammation, evaluating the expression of MMP1, MMP3, TGFβ and TIMP-1 by real-time PCR. Patients were also evaluated up to two years’ follow-up by using: VAS for pain, IKDC-subjective and KOOS scores.
Results
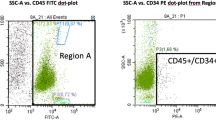
The laboratory analysis showed that BMSCs accounted for 0.011% of BMA cells, similar to what had been expected in native bone marrow. The paracrine activity of BMA was able to reduce in vitro the catabolic response of human chondrocyte, as shown by the decrease in metalloproteases concentration and increase in anti-inflammatory mediators. Moreover, the clinical evaluation showed significant improvements in all scores adopted, with stable results up to two years.
Conclusion
The present data showed the effectiveness of the study device to harvest pure bone marrow with minimal peripheral blood contamination. The relevant content of MSCs resulted in the ability to counteract the catabolic cascade through a paracrine action. The clinical outcomes in patients affected by unicompartmental knee OA were encouraging in terms of pain reduction and functional improvement up to mid-term evaluation.





Similar content being viewed by others
Data availability
Data are available from the corresponding Author upon reasonable request.
Code availability
NA.
Change history
19 January 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00264-021-05297-7
References
Cavallo C, Boffa A, Andriolo L et al (2021) Bone marrow concentrate injections for the treatment of osteoarthritis: evidence from preclinical findings to the clinical application. Int Orthop (SICOT) 45:525–538. https://doi.org/10.1007/s00264-020-04703-w
Imam MA, Holton J, Ernstbrunner L et al (2017) A systematic review of the clinical applications and complications of bone marrow aspirate concentrate in management of bone defects and nonunions. Int Orthop 41:2213–2220. https://doi.org/10.1007/s00264-017-3597-9
Palombella S, Lopa S, Gianola S et al (2019) Bone marrow-derived cell therapies to heal long-bone nonunions: a systematic review and meta-analysis—which is the best available treatment? Stem Cells Int 2019:e3715964. https://doi.org/10.1155/2019/3715964
Sampson S, Botto-van Bemden A, Aufiero D (2013) Autologous bone marrow concentrate: review and application of a novel intra-articular orthobiologic for cartilage disease. Phys Sportsmed 41:7–18. https://doi.org/10.3810/psm.2013.09.2022
Murphy MB, Moncivais K, Caplan AI (2013) Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 45:e54. https://doi.org/10.1038/emm.2013.94
Colombini A, Perucca Orfei C, Kouroupis D et al (2019) Mesenchymal stem cells in the treatment of articular cartilage degeneration: new biological insights for an old-timer cell. Cytotherapy 21:1179–1197. https://doi.org/10.1016/j.jcyt.2019.10.004
Murphy EP, Fenelon C, McGoldrick NP, Kearns SR (2018) Bone marrow aspirate concentrate and microfracture technique for talar osteochondral lesions of the ankle. Arthrosc Tech 7:e391. https://doi.org/10.1016/j.eats.2017.10.011
Kreuz PC, Steinwachs MR, Erggelet C et al (2006) Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage 14:1119–1125. https://doi.org/10.1016/j.joca.2006.05.003
Wong KL, Lee KBL, Tai BC et al (2013) Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthroscopy 29:2020–2028. https://doi.org/10.1016/j.arthro.2013.09.074
Shin Y-S, Yoon J-R, Kim H-S, Lee S-H (2018) Intra-articular injection of bone marrow-derived mesenchymal stem cells leading to better clinical outcomes without difference in MRI outcomes from baseline in patients with knee osteoarthritis. Knee Surg Relat Res 30:206–214. https://doi.org/10.5792/ksrr.17.201
Ganguly P, El-Jawhari JJ, Giannoudis PV et al (2017) Age-related changes in bone marrow mesenchymal stromal cells: a potential impact on osteoporosis and osteoarthritis development. Cell Transplant 26(9):1520–1529. https://doi.org/10.1177/0963689717721201
Schäfer R, DeBaun MR, Fleck E et al (2019) Quantitation of progenitor cell populations and growth factors after bone marrow aspirate concentration. J Transl Med 17:115. https://doi.org/10.1186/s12967-019-1866-7
Narbona-Carceles J, Vaquero J, Suárez-Sancho SBS et al (2014) Bone marrow mesenchymal stem cell aspirates from alternative sources: is the knee as good as the iliac crest? Injury 45(Suppl 4):S42-47. https://doi.org/10.1016/S0020-1383(14)70009-9
Hernigou P, Homma Y, Flouzat Lachaniette CH et al (2013) Benefits of small volume and small syringe for bone marrow aspirations of mesenchymal stem cells. Int Orthop 37:2279–2287. https://doi.org/10.1007/s00264-013-2017-z
Chong P-P, Selvaratnam L, Abbas AA, Kamarul T (2012) Human peripheral blood derived mesenchymal stem cells demonstrate similar characteristics and chondrogenic differentiation potential to bone marrow derived mesenchymal stem cells. J Orthop Res 30:634–642. https://doi.org/10.1002/jor.21556
Friedenstein AJ, Latzinik NW, Grosheva AG, Gorskaya UF (1982) Marrow microenvironment transfer by heterotopic transplantation of freshly isolated and cultured cells in porous sponges. Exp Hematol 10:217–227
Wexler SA, Donaldson C, Denning-Kendall P et al (2003) Adult bone marrow is a rich source of human mesenchymal “stem” cells but umbilical cord and mobilized adult blood are not. Br J Haematol 121:368–374. https://doi.org/10.1046/j.1365-2141.2003.04284.x
Krause DS, Fackler MJ, Civin CI, May WS (1996) CD34: structure, biology, and clinical utility. Blood 87:1–13
Varady NH, Cate G, Barghi A et al (2020) Positive early clinical outcomes of bone marrow aspirate concentrate for osteoarthritis using a novel fenestrated trocar. Knee 27:1627–1634. https://doi.org/10.1016/j.knee.2020.08.018
Lv F-J, Tuan RS, Cheung KMC, Leung VYL (2014) Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells 32:1408–1419. https://doi.org/10.1002/stem.1681
Han Y, Li X, Zhang Y et al (2019) Mesenchymal stem cells for regenerative medicine. Cells 8(8):886. https://doi.org/10.3390/cells8080886
Scarpone M, Kuebler D, Chambers A et al (2019) Isolation of clinically relevant concentrations of bone marrow mesenchymal stem cells without centrifugation. J Transl Med 17:10. https://doi.org/10.1186/s12967-018-1750-x
Hegde V, Shonuga O, Ellis S et al (2014) A prospective comparison of 3 approved systems for autologous bone marrow concentration demonstrated nonequivalency in progenitor cell number and concentration. J Orthop Trauma 28:591–598. https://doi.org/10.1097/BOT.0000000000000113
McLain RF, Fleming JE, Boehm CA, Muschler GF (2005) Aspiration of osteoprogenitor cells for augmenting spinal fusion: comparison of progenitor cell concentrations from the vertebral body and iliac crest. J Bone Joint Surg Am 87:2655–2661. https://doi.org/10.2106/JBJS.E.00230
De Luca P, Kouroupis D, Viganò M et al (2019) Human diseased articular cartilage contains a mesenchymal stem cell-like population of chondroprogenitors with strong immunomodulatory responses. J Clin Med 8:423. https://doi.org/10.3390/jcm8040423
Wang M, Zhou Y, Huang W et al (2020) Association between matrix metalloproteinase-1 (MMP-1) protein level and the risk of rheumatoid arthritis and osteoarthritis: a meta-analysis. Braz J Med Biol Res 54:e10366. https://doi.org/10.1590/1414-431X202010366
Zhou L, Ye H, Liu L, Chen Y (2021) Human Bone mesenchymal stem cell-derived exosomes inhibit IL-1β-induced inflammation in osteoarthritis chondrocytes. Cell J 23:485–494. https://doi.org/10.22074/cellj.2021.7127
Gato-Calvo L, Hermida-Gómez T, Romero CR et al (2019) Anti-inflammatory effects of novel standardized platelet rich plasma releasates on knee osteoarthritic chondrocytes and cartilage in vitro. Curr Pharm Biotechnol 20:920–933. https://doi.org/10.2174/1389201020666190619111118
Yang J, Guo A, Li Q, Wu J (2021) Platelet-rich plasma attenuates interleukin-1β-induced apoptosis and inflammation in chondrocytes through targeting hypoxia-inducible factor-2α. Tissue Cell 73:101646. https://doi.org/10.1016/j.tice.2021.101646
Dabrowski MP, Stankiewicz W, Płusa T et al (2001) Competition of IL-1 and IL-1ra determines lymphocyte response to delayed stimulation with PHA. Mediators Inflamm 10:101–107
Ortiz LA, DuTreil M, Fattman C et al (2007) Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A 104:11002–11007. https://doi.org/10.1073/pnas.0704421104
Caplan AI, Correa D (2011) The MSC: an injury drugstore. Cell Stem Cell 9:11–15. https://doi.org/10.1016/j.stem.2011.06.008
Matteo BD, Filardo G, Kon E, Marcacci M (2015) Platelet-rich plasma: evidence for the treatment of patellar and Achilles tendinopathy—a systematic review. Musculoskelet Surg 99(1):1–9. https://doi.org/10.1007/s12306-014-0340-1
Liu Z, Simpson RJ, Cheers C (1995) Interaction of interleukin-6, tumour necrosis factor and interleukin-1 during Listeria infection. Immunology 85:562–567
Khella CM, Asgarian R, Horvath JM et al (2021) An evidence-based systematic review of human knee Post-Traumatic Osteoarthritis (PTOA): timeline of clinical presentation and disease markers, comparison of knee joint PTOA models and early disease implications. Int J Mol Sci 22(4):1996. https://doi.org/10.3390/ijms22041996
Wiegertjes R, Thielen NGM, van Caam APM et al (2021) Increased IL-6 receptor expression and signaling in ageing cartilage can be explained by loss of TGF-β-mediated IL-6 receptor suppression. Osteoarthr Cartil. https://doi.org/10.1016/j.joca.2021.01.008
Viganò M, Lugano G, Perucca Orfei C et al (2019) Autologous microfragmented adipose tissue reduces the catabolic and fibrosis response in an in vitro model of tendon cell inflammation. Stem Cells Int 2019:5620286. https://doi.org/10.1155/2019/5620286
Chahla J, Dean CS, Moatshe G et al (2016) Concentrated bone marrow aspirate for the treatment of chondral injuries and osteoarthritis of the knee: a systematic review of outcomes. Orthop J Sports Med 4:2325967115625481. https://doi.org/10.1177/2325967115625481
Colberg RE, Jurado Vélez JA, Walsh KP, Fleisig G (2020) Short-term outcomes after pure bone marrow aspirate injection for severe knee osteoarthritis: a case series. Regen Med 15:1851–1859. https://doi.org/10.2217/rme-2019-0113
Acknowledgements
The authors wish to thank Dr. Federico Valli, Dr. Paola De Luca, Dr. Elena De Vecchi and Dr. Agostino La Porta for their support to the study.
Funding
The present research was supported by: the Italian Ministry of Health “Ricerca Corrente”, Geistlich Biomaterials Italia Srl, and a grant from the ‘ON Foundation’, Switzerland (ON project number: 18–054).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The pilot clinical study was approved by the Hospital Ethics Committee and Internal Review Board of IRCCS Humanitas Research Center (protocol number: 530/18, approved on 18th September 2018).
Consent for publication
NA
Conflict of interest
The authors declare no personal conflict of interests. Geistlich Biomaterials Srl provided part of the funding for the present research.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised. The correct copyright should be “The Author(s) under exclusive licence to SICOT aisbl.”
Rights and permissions
About this article
Cite this article
Viganò, M., Ragni, E., Di Matteo, B. et al. A single step, centrifuge-free method to harvest bone marrow highly concentrated in mesenchymal stem cells: results of a pilot trial. International Orthopaedics (SICOT) 46, 391–400 (2022). https://doi.org/10.1007/s00264-021-05243-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-021-05243-7