Abstract
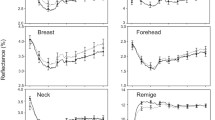
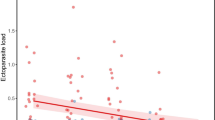
Parents are expected to strategically partition their limited resources among the current and future progeny in order to maximize their fitness. Since an equal investment in offspring of different reproductive value entails fitness costs, natural selection has promoted the evolution of reliable signals of offspring condition, allowing parents to invest in their progeny accordingly. In birds, mouth and skin colouration are hypothesized to be honest signals of offspring condition, because they are affected by diverse factors. Among these, ectoparasite load has been shown to affect nestling condition, but its influence on visual components of begging is poorly known. We experimentally investigated whether nest ectoparasite removal affected flange and skin reflectance of first- and second-brood European starling (Sturnus vulgaris) nestlings. We also tested whether high reflectance in visual components of begging mirrored other aspects of nestling condition, such as morphological (high stature) and physiological (high haematocrit and immune response) traits, and pre-fledging mortality. Ectoparasite removal did not affect visual components of begging in first or second broods. However, larger nestlings from both broods displayed higher ultraviolet (UV) reflectance of skin and higher flange reflectance in the visible-wavelength region (but lower flange UV reflectance) than their siblings. A higher skin UV reflectance relative to siblings also positively predicted pre-fledging survival within-brood. Therefore, visual components of begging did not mirror ectoparasite infestation in this species. However, they provide parents with reliable information about individual quality, thus affecting resource allocation and promoting survival of the most valuable offspring during the entire breeding season.
Significance statement
In species with parental care, natural selection favours the evolution of reliable signals of offspring quality, thus allowing parents to invest in their progeny accordingly. We experimentally show that skin and beak flange colour does not mirror ectoparasite infection in European starling nestlings. However, begging visual signals predict nestling body size and survival until fledging. A seasonal variation in the strength of the association between begging visual signals and nestling condition is also shown, indicating that change ecological conditions can affect the association between different condition-dependent traits.

Similar content being viewed by others
References
Arnold KE, Ramsay SL, Henderson L, Larcombe SD (2010) Seasonal variation in diet quality: antioxidants, invertebrates and blue tits Cyanistes caeruleus. Biol J Linn Soc 99:708–717
Avery ML, Primus TM, Mihaich EM, Decker DG, Humphrey JS (1998) Consumption of fipronil-treated rice seed does not affect captive blackbirds. Pestic Sci 52:91–96
de Ayala RM, Saino N, Møller AP, Anselmi C (2007) Mouth coloration of nestlings covaries with offspring quality and influences parental feeding behaviour. Behav Ecol 18:26–534
Badyaev AV, Hamstra TL, Oh KP, Seaman DAA (2006) Sex-biased maternal effects reduce ectoparasite-induced mortality in a passerine bird. P Natl Acad Sci USA 103:14406–14411
Bize P, Piault R, Moureau B, Heeb P (2006) A UV signal of offspring condition mediates context-dependent parental favouritism. Proc R Soc Lond B 273:2063–2068
Brown CR, Brown MB (1992) Ectoparasitism as a cause of natal dispersal in cliff swallows. Ecology 73:1718–1723
Candolin U (2000) Changes in expression and honesty of sexual signalling over the reproductive lifetime of sticklebacks. Proc R Soc Lond B 267:2425–2430
Cantarero A, López-Arrabé J, Redondo AJ, Moreno J (2013) Behavioural responses to ectoparasites in pied flycatchers Ficedula hypoleuca: an experimental study. J Avian Biol 44:591–599
Caro SM, Griffin AS, Hinde CA, West SA (2016) Unpredictable environments lead to the evolution of parental neglect in birds. Nat Commun 7:10985
Christe P, Richner H, Oppliger A (1996) Begging, food provisioning, and nestling competition in great tit broods infested with ectoparasites. Behav Ecol 7:127–131
Clark L, Mason JR (1988) Effect of biologically active plants used as nest material and the derived benefit to starling nestlings. Oecologia 77:174–180
Costantini D, Møller AP (2008) Carotenoids are minor antioxidants for birds. Funct Ecol 22:367–370
Cotton S, Fowler K, Pomiankowski A (2004) Do sexual ornaments demonstrate heightened condition-dependent expression as predicted by the handicap hypothesis? Proc R Soc Lond B 271:771–783
Cramp S (1998) The complete birds of the western Palearctic on CD-ROM. Oxford University Press, Oxford
Davis JN, Todd PM, Bullock S (1999) Environment quality predicts parental provisioning decisions. Proc R Soc Lond B 266:1791–1797
Dawson RD, Bortolotti GR (1997) Are avian hematocrits indicative of condition? American kestrels as a model. J Wildlife Manage 61:1297–1306
Doutrelant C, Grégoire A, Grnac N, Gomez D, Lambrechts MM, Perret P (2008) Female coloration indicates female reproductive capacity in blue tits. J Evol Biol 21:226–233
Dugas MB (2009) House sparrow (Passer domesticus) parents preferentially feed nestlings with mouth colours that appear carotenoid-rich. Anim Behav 78:767–772
Dugas MB (2012) Cross-fostering reveals that among-brood differences in ornamental mouth colouration mostly reflect rearing conditions in nestling house sparrows. Biol J Linn Soc 106:169–179
Dugas MB (2015) Commentary: parental care and the proximate links between maternal effects and offspring fitness. Oecologia 177:1089–1092
Dugas MB, Doumas LT (2014) Ectoparasite density is associated with mouth colour and size in nestling house sparrows Passer domesticus. Ibis 156:682–686
Dugas MB, McGraw KJ (2011) Proximate correlates of carotenoid-based mouth coloration in nestlings house sparrow. Condor 113:691–700
Dugas MB, Rosenthal GG (2010) Carotenoid-rich mouth colors influence the conspicuousness of nestling birds. Behav Ecol Sociobiol 64:455–462
Ewen GE, Thorogood R, Karadas F, Cassey P (2008) Condition dependence of nestling mouth colour and effect of supplementing carotenoids on parental behaviour in the hihi (Notiomystis cinta). Oecologia 157:361–368
Fair J, Whitaker S, Pearson B (2007) Sources of variation in haematocrit in birds. Ibis 149:535–552
Gil D, Bulmer E, Celis P, Lopez-Rull I (2008) Adaptive developmental plasticity in growing nestlings: sibling competition induces differential gape growth. Proc R Soc Lond B 275:549–554
Godfray HCJ (1991) Signaling of need by offspring to their parents. Nature 352:328–330
Griffiths R, Double MC, Orr K, Dawson RJ (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075
Griggio M, Morosinotto C, Pilastro A (2009) Nestlings’ carotenoid feather ornament affects parental allocation strategy and reduces maternal survival. J Evol Biol 22:2077–2085
Gwinner H, Oltrogge M, Trost L, Nienaber U (2000) Green plants in starling nests: effects on nestlings. Anim Behav 59:301–309
Hartley RC, Kennedy MW (2004) Are carotenoids a red herring in sexual display? Trends Ecol Evol 19:353–354
Hill G, McGraw K (2006) Bird coloration. Mechanisms and measurements. Harvard University Press, Cambridge Vol. 1
Hunt S, Kilner RM, Langmore NE, Bennett ATD (2003) Conspicuous, ultraviolet-rich mouth colours in begging chicks. Proc R Soc Lond B 270:S25–S28
Jacob S, Heeb P (2013) Mouth colour components of begging are dynamic signals of quality in European starling nestlings. J Avian Biol 44:39–44
Jacob S, Rieucau G, Heeb P (2011) Multimodal begging signals reflect independent indices of nestling condition in European starlings. Behav Ecol 22:1249–1255
Jacob S, Parthuisot N, Vallat A, Ramon-Portugal F, Helfenstein F, Heeb P (2015) Microbiome affects egg carotenoid investment, nestling development and adult oxidative costs of reproduction in great tits. Funct Ecol 29:1048–1058
Johnstone RA (1999) Signalling of need, sibling competition and the cost of honesty. P Natl Acad Sci USA 96:12644–12649
Jourdie V, Moureau B, Bennet ATD, Heeb P (2004) Ultraviolet reflectance by the skin of nestlings. Nature 431:262
Kacelnik A, Cotton PA, Stirling L, Wright J (1995) Food allocation among nestling starlings: sibling competition and the scope of parental choice. Philos T Roy Soc B 259:259–263
Kilner RM (1997) Mouth colour is a reliable signal of need in begging canary nestlings. Proc R Soc Lond B 264:963–968
Kilner RM (2002) The evolution of complex begging displays. In: Wright J, Leonard ML (eds) The evolution of begging: competition, cooperation and communication. Kluwer Academic, Dordrecht, pp 87–106
Kilner RM, Johnstone RA (1997) Begging the question: are offspring solicitation behaviours signals of need? Trends Ecol Evol 12:11–15
Leonard ML, Horn AG, Parks E (2003) The role of posturing and calling in the begging display of nestling birds. Behav Ecol Sociobiol 54:188–193
Lessells CM (2002) Parentally biased favouritism: why should parents specialize in caring for different offspring? Philos T Roy Soc B 357:381–403
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 1:116–121
Liker A, Márkus M, Vozár Á, Zemankovics E, Rózsa L (2001) Distribution of Carnus hemapterus in a starling colony. Can J Zool 79:574–580
Lindström J (1999) Early development and fitness in birds and mammals. Trends Ecol Evol 14:343–348
Loiseau C, Fellous S, Haussy C (2008) Condition-dependent effects of corticosterone on a carotenoid-based begging signal in house sparrows. Horm Behav 53:266–273
Lyon BE, Eadie JM, Hamilton LD (1994) Parental choice selects for ornamental plumage in American coot chicks. Nature 371:240–243
Marri V, Richner H (2014) Yolk carotenoids increase fledging success in great tit nestlings. Oecologia 176:371–377
Martínez-de la Puente J, Merino S, Tomás G, Moreno J, Morales J, Lobato E, Martínez J (2011) Nest ectoparasites increase physiological stress in breeding birds: an experiment. Naturwissenschaften 98:99–106
Mazgajski TD (2007) Effect of old nest material in nestboxes on ectoparasite abundance and reproductive output in the European Starling Sturnus vulgaris. Pol J Ecol 55:377–385
Merino S, Potti J (1995) Mites and blowflies decrease growth and survival in nestling pied flycatchers. Oikos 73:95–103
Mock DW, Parker GA (1997) The evolution of sibling rivalry. Oxford University Press, New York
Mock DW, Dugas MB, Strickler SA (2011) Honest begging: expanding from signal of need. Behav Ecol 22:909–917
Møller AP (1989) Parasites, Predators and nest boxes: Facts and artefacts in nest box studies of birds? Oikos 56:421–423
Møller AP (1993) Ectoparasites increase the cost of reproduction in their hosts. J Anim Ecol 62:309–322
Møller AP, Erritzøe J (1996) Parasite virulence and host immune defense: host immune response is related to nest reuse in birds. Evolution 50:2066–2072
Møller AP, Arriero E, Lobato E, Merino S (2009) A meta-analysis of parasite virulence in nestling birds. Biol Rev 84:567–588
Moreno-Rueda G, Redondo T, Ochoa D, Camacho C, Canal D, Potti J (2016) Nest-dwelling ectoparasites reduce begging effort in pied flycatcher Ficedula hypoleuca nestlings. Ibis 158:881–886
O’Connor JA, Sulloway FJ, Robertson J, Kleindorfer S (2010) Philornis downsi parasitism is the primary cause of nestling mortality in the critically endangered Darwin’s medium tree finch (Camarhynchus pauper). Biodivers Conserv 19:853–866
Olson VA, Owens IPF (1998) Costly sexual signals: are carotenoids rare, risky or required? Trends Ecol Evol 12:510–514
Parker GA, Royle NJ, Hartley IR (2002) Intrafamilial conflict and parental investment: a synthesis. Philos T Roy Soc B 357:295–307
Pinxten R, Eens M, Verheyen RF (1990) Intermediate clutches in the Starling (Sturnus vulgaris): replacement clutches, additional clutches of polygynous males or late first clutches? J Ornithol 131:141–150
Pirrello S, Pilastro A, Serra L (2015) Nest-dwelling ectoparasites influence the start and duration of the first pre-basic moult in the European starling Sturnus vulgaris. J Avian Biol 46:412–418
van de Pol M, Wright J (2009) A simple method for distinguishing within- versus between-subject effects using mixed models. Anim Behav 77:753–758
Powlesland RG (1977) Effects of the haematophagous mite Ornithonyssus bursa on nestling starlings in New Zealand. New Zeal J Zool 4:85–94
Romano A, Rubolini D, Caprioli M, Boncoraglio G, Ambrosini R, Saino N (2011) Sex-related effects of an immune challenge on growth and begging behavior of barn swallow nestlings. PLoS One 6:e22805
Romano A, Bazzi G, Caprioli M, Corti M, Costanzo A, Rubolini D, Saino (2016) Nestling sex and plumage color predict food allocation by barn swallow parents. Behav Ecol 27:1198–1205
Royle NJ, Smiseth PT, Kölliker M (2012) The evolution of parental care. Oxford University Press, Oxford
Ruiz-Castellano C, Soler M, Rösler A, Martín-Gálvez D, Soler JJ (2016) Context-dependent effects of an experimental increase of hunger level in house sparrow nestlings. Behav Ecol Sociobiol 70:939–949
Saino N, Ninni P, Calza S, Martinelli R, De Bernardi F, Møller AP (2000) Better red than dead: corotenoid based mouth coloration reveals infection in barn swallow nestlings. Proc R Soc Lond B 267:57–61
Saino N, Romano M, Scandolara C, Rubolini D, Ambrosini R, Caprioli M, Costanzo A, Romano A (2014) Brownish, small and lousy barn swallows have greater natal dispersal propensity. Anim Behav 87:137–146
SAS Institute (2006) The GLIMMIX procedure. SAS Institute, Cary, NC
Serra L, Pirrello S, Caprioli M et al. (2012) Seasonal decline of off spring quality in the European starling Sturnus vulgaris: an immune challenge experiment. Behav Ecol Sociobiol 66:697–709
Seth A, Pradhan S, Purkait S, Sinha S, Chowdhury A (2016) Effects of fipronil, a pyrazole insecticide, on microbial biomass carbon, soil respiration, FDA and dehydrogenase activity of soil. Int J Adv Biol Res 6:352–356
Soler JJ, Avilés JM, Cuervo JJ, Perez-Contreras T (2007) Is the relation between colour and immune response mediated by nutritional condition in spotless starling nestlings? Anim Behav 74:1139–1145
Svensson PA, Wong BBM (2011) Carotenoid-based signals in behavioural ecology: a review. Behaviour 148:131–189
Szép T, Møller AP (1999) Cost of parasitism and host immune defence in the sand martin Riparia riparia: a role for parent-off spring conflict? Oecologia 119:9–15
Tella JL, Lemus JA, Carrete M, Blanco G (2008) The PHA test reflects acquired T-cell mediated immunocompetence in birds. PLoS One 3:e3295
Thorogood R, Kilner RM, Karadas F, Ewen JG (2008) Spectral mouth colour of nestlings changes with carotenoid availability. Funct Ecol 22:1044–1051
Tomás G, Merino S, Martínez-de la Puente J, Moreno J, Morales J, Lobato E (2008) Determinants of abundance and effects of blood-sucking flying insects in the nest of a hole-nesting bird. Oecologia 156:305–312
Trivers RL (1974) Parent-offspring conflict. Am Zool 14:249–264
Tschirren B, Fitze PS, Richner H (2003) Proximate mechanisms of variation in the carotenoid-based plumage coloration of nestling great tits (Parus major L.) J Evol Biol 16:91–100
Vergara P, Martinez-Padilla J, Mougeot F, Leckie F, Redpath SM (2012) Environmental heterogeneity influences the reliability of secondary sexual traits as condition indicators. J Evol Biol 25:20–28
Verhulst S, Nilsson JÅ (2008) The timing of birds’ breeding seasons: a review of experiments that manipulated timing of breeding. Philos T Roy Soc B 363:399–410
Wright J, Leonard ML (2002) The evolution of begging: competition, cooperation and communication. Kluwer Academic, Dordrecht, The Netherlands
Acknowledgements
We are grateful to L. Zangari and G. Viscardi who greatly helped during field work. Three anonymous referees also provided very useful comments to previous versions of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This research was undertaken (capture and experimental treatments) under the combined prescriptions of Art. 4 (1) and Art. 7 (5) of the Italian law 157/1992, which regulates studies on wild bird species. This law also regulates taking, keeping and manipulating procedures to be followed for the study of wild birds. Standard procedures for capturing and handling nestlings in the nest-box were used. The duration of handling nestlings was kept to a minimum to minimize stress.
Funding
SP and AP were partially supported by a grant from the Fondazione CARIPARO (Progetto di eccellenza 2007). AR was funded by the Postdoctoral Fellowship Program of the Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by K. McGraw
Electronic supplementary material
ESM 1
(DOCX 40 kb)
Rights and permissions
About this article
Cite this article
Pirrello, S., Colombo, E., Pilastro, A. et al. Skin and flange colour, but not ectoparasites, predict condition and survival in starling nestlings. Behav Ecol Sociobiol 71, 63 (2017). https://doi.org/10.1007/s00265-017-2292-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-017-2292-6