Abstract
In ants, bees, and other social Hymenoptera, alarm pheromones are widely employed to coordinate colony nest defense. In that context, alarm pheromones elicit innate species-specific defensive behaviors. Therefore, in terms of classical conditioning, an alarm pheromone could act as an unconditioned stimulus (US). Here, we test this hypothesis by establishing whether repeated exposure to alarm pheromone in different testing contexts modifies the alarm response. We evaluate colony-level alarm responses in the stingless bee, Tetragonisca angustula, which has a morphologically distinct guard caste. First, we describe the overall topology of defense behaviors in the presence of an alarm pheromone. Second, we show that repeated, regular exposure to synthetic alarm pheromone reduces different components of the alarm response, and memory of that exposure decays over time. This observed decrease followed by recovery occurs over different time frames and is consistent with behavioral habituation. We further tested whether the alarm pheromone can act as a US to classically condition guards to modify their defense behaviors in the presence of a novel (conditioned) stimulus (CS). We found no consistent changes in the response to the CS. Our study demonstrates the possibility that colony-level alarm responses can be adaptively modified by experience in response to changing environmental threats. Further studies are now needed to reveal the extent of these habituation-like responses in regard to other pheromones, the potential mechanisms that underlie this phenomenon, and the range of adaptive contexts in which they function at the colony level.
Significance statement
Pheromones are classically thought to elicit stereotyped action patterns. Here, we test the idea that responses to pheromones are plastic and show characteristics of an unconditioned stimulus. This study demonstrates clear non-associative plasticity in the colony-level response to alarm pheromone, in the stingless honey bee, Tetragonisca angustula. Colonies of T. angustula show habituation-like responses across multiple measures to repeated stimulation of their alarm pheromone. We therefore demonstrate that colony-level responses to pheromones are adaptively plastic. Finally, we failed to demonstrate colony-level conditioning using alarm pheromone as the unconditioned stimulus; however, these findings and others warrant further investigation.






Similar content being viewed by others
References
Bicker G, Hähnlein I (1994) Long-term habituation of an appetitive reflex in the honeybee. Neuroreport 6(1):54–56
Birgiolas J, Jernigan CM, Smith BH, Crook S (2016) SwarmSight: measuring the temporal progression of animal group activity levels from natural scene and laboratory videos. Behav Res 49:576–587. https://doi.org/10.3758/s13428-016-0732-2
Blum MS (1969) Alarm pheromones. Annu Rev Entomol 14:57–80
Bowden RM, Garry MF, Breed MD (1994) Discrimination of con- and heterospecific bees by Trigona (Tetragonisca) angustula guards. J Kansas Entomol Soc 67(1):137–139
Butler CG, Free JB (1952) The behavior of worker honeybees at the hive entrance. Behaviour 4:263–291
Chaput M, Panhuber H (1982) Effects of long duration odor exposure on the unit activity of olfactory bulb cells in awake rabbits. Brain Res 250:41–52
Colbert HA, Bargmann CI (1995) Odorant-specific adaptation pathways generate olfactory plasticity in C. elegans. Neuron 14:803–812
Cunningham JP, Hereward JP, Heard TA, De Barro PJ, West SA (2014) Bees at war: interspecific battles and nest usurpation in stingless bees. Am Nat 184(6):777–786. https://doi.org/10.1086/678399
Dawson EH, Chittka L, Leadbeater E (2016) Alarm substances induce associative social learning in honeybees, Apis mellifera. Anim Behav 122:17–22. https://doi.org/10.1015/j.anbehav.2016.08.006
Francke W, Lübke G, Schröder W, Reckziegel A, Imperatriz-Fonseca V, Kleinert A, Engels E, Hartfelder K, Radtke R, Engels W (2000) Identification of oxygen containing volatiles in cephalic secretions of workers of Brazilian stingless bees. J Braz Chem Soc 11(6):562–571
Gong Z, Wang C, Dong S, Zhang X, Wang Y, Hu Z, Tan K (2017) High concentrations of the alarm pheromone component, isopentyl acetate, reduces foraging and dancing in Apis mellifera Ligustica and Apis cerana Cerana. J Insect Behav 30:188–198. https://doi.org/10.1007/s10905-017-9606-4
Grosso AF, Bego LR (2002) Labor division, average life span, survival curve, and nest architecture of Tetragonisca angustula angustula (Hymenoptera, Apinae, Meliponini). Sociobiology 40(3):615–637
Grüter C, Kärcher MH, Ratnieks FLW (2011) The natural history of nest defense in a stingless bee, Tetragonisca angustula (Latreille) (Hymenoptera: Apidae), with two distinct types of entrance guards. Neotrop Entomol 40(1):55–61
Grüter C, Menezes C, Imperatriz-Fonseca VL, Ratnieks FLW (2012) A morphologically specialized soldier caste improves colony defense in a neotropical eusocial bee. Proc Natl Acad Sci 109(4):1182–1186
Grüter C, von Zuben LG, Segers FHID, Cunningham JP (2016) Warfare in stingless bees. Insect Soc:1–14. https://doi.org/10.1007/s00040-0160468-0
Grüter C, Segers FHID, Santos LLG, Hammel B, Zimmermann U, Nascimento FS (2017a) Enemy recognition is linked to soldier size in a polymorphic stingless bee. Biol Lett 13:20170511. https://doi.org/10.1098/rsbl.2017.05511
Grüter C, Segers FHID, Menezes C, Vollet-Neto A, Falcón T, von Zuben L, Bitondi MMG, Nascimento FS, Almeida EAB (2017b) Repeated evolution of soldier sub-castes suggests parasitism drives social complexity in stingless bees. Nat Commun 8(4):1–7. https://doi.org/10.1038/s41467-016-0012-y
Hammel B, Vollet-Neto A, Menezes C, Nascimento FS, Engels, Grüter C (2016) Soldiers in a stingless bee: work rate and task repertoire suggest they are an elite force. Am Nat 187(1):120–129
Hansel MH (1993) The ecological impact of animal nests and burrows. Funct Ecol 7:5–12
Harris JD (1943) Habituatory response decrement in the intact organism. Psychol Bull 40(6):385–422
Hermann HR (1971) Sting autotomy—defense mechanism in certain social hymenoptera. Insect Soc 18(2):111–120
Kaissling KE, Strausfeld C, Rumbo E (1987) Adaptation processes in insect olfactory receptors. Ann N Y Acad Sci 510:104–112
Kärcher MH, Ratnieks FLW (2009) Standing and hovering guards of the stingless bee Tetragonisca angustula compliment each other in entrance guarding and intruder recognition. J Apic Res 48:209–214
Kelber A, Ziel J (1990) A robust procedure for visual stabilization of hovering flight position in guard bees of Trigona (Tetragonisca) angustula (Apidae, Meliponinae). J Comp Physiol A 167:569–577
Kelling F, Ialenti F, Den Otter C (2002) Background odour induces adaptation and sensitization of olfactory receptors in the antennae of houseflies. Med Vet Entomol 16:161–169
Kurashashi T, Menini A (1997) Mechanism of odorant adaptation in the olfactory receptor cell. Nature 395(6618):725–729. https://doi.org/10.1038/385725a0
Makintosh NJ (1985) Conditioning and associative learning. Oxford University Press, New York
Martínez-Ricós J, Agustín-Pavón C, Lanuza E, Martínez-García F (2007) Intraspecific communication through chemical signals in female mice: reinforcing properties of involatile male sexual pheromones. Chem Senses 32:139–148
Maschwitz U (1964) Gefahrenalarmstoffe und gefahrenalarmierung bei sozialen hymenopteran. Zeitschrift für vergleichende Physiologie 47:596–665
Maschwitz UW (1966) Alarm substances and alarm behavior in social insects. Vitam Horm 24:267–290
McGlynn TP (2012) The ecology of nest movement in social insects. Annu Rev Entomol 57:291–308
Menzel R (1999) Memory dynamics in the honeybee. J Comp Physiol A 185:323–340
Moore D, Angel JE, Cheesman IM, Fahrbach SE, Robinson G (1998) Timekeeping in the honey bee colony: integration of circadian rhythms and division of labor. Behav Ecol Sociobiol 43:147–160
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/.
Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton DF, Clombo J, Coppola G, Geyer MA, Glanzman DL, Marsland S, McSweeney FK, Wison DA, Wu C, Thompson RF (2009) Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiol Learn Mem 92(2):135–138. https://doi.org/10.1016/j.nlm.2008.09.012
Rescorla RA (1988) Behavioral studies of pavlovian conditioning. Ann Rev Neurosci 11:329–352
Roubik DW (1989) Ecology and natural history of tropical bees. Cambridge University Press, New York
Roubik DW (2006) Stingless bee nesting biology. Apidologie 27:124–143
Sakagami SF, Roubik DW, Zucchi R (1993) Ethology of the robber stingless bee, Lestrimelitta limao (Hymenoptera: Apidae). Sociobiology 21:147–166
Sasaki T, Hölldobler B, Millar JG, Pratt SC (2014) A context-dependent alarm signal in the ant Temnothorax rugatulus. J Exp Biol 217:3229–3236
Shackleton K, Al Toufailia H, Balfour NJ, Nascimento FS, Alves DA, Ratnieks FL (2015) Appetite for self-destruction: suicidal biting as a nest defense strategy in Trigona stingless bees. Behav Ecol Sociobiol 69:273–281
Shorey HH (1973) Behavioral responses to insect pheromones. Annu Rev Entomol 18:349–380
Smith BH, Roubik DW (1983) Mandibular glands of stingless bees (Hymenoptera: Apidae): chemical analysis of their contents and biological function in two species of Melipona. J Chem Ecol 9(11):1465–1472
Störtkuhl KF, Hovemann BT, Carlson JR (1999) Olfactory adaptation depends on the Trp Ca2+ channel in Drosophila. J Neurosci 19:4839–4846
Tan K, Dong S, Li X, Liu X, Wang C, Li J, Nieh JC (2016) Honey bee inhibitory signaling is tuned to threat severity and can act as a colony alarm signal. PLoS Biol 14(3):e1002423. https://doi.org/10.1371/journal.pbio.1002423
Thompson RF, Spencer WA (1966) Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev 73(1):16–43
Troen H, Dubrovsky I, Tamir R, Bloch G (2008) Temporal variation in group aggressiveness of honeybee (Apis mellifera) guards. Apidologie 39:283–291
Tully T, Preat T, Boynton SC, Del Vecchio M (1994) Genetic dissection of consolidated memory in Drosophila. Cell 79:35–47
Urlacher E, Francés B, Giurfa M, Devaud J-M (2010) An alarm pheromone modulates appetitive olfactory learning in the honeybee (Apis mellifera). Front Behav Neurosci 4:157. https://doi.org/10.3389/fnbeh.2010.00157
van Zweden JS, Grüter C, Jones SM, Ratnieks FLW (2011) Hovering guards of the stingless bee Tetragonisca angustula increase colony defensive perimeter as shown by intra- and inter-specific comparisons. Behav Ecol Sociobiol 65:1277–1282
von Zuben LG, Schorkopf DLP, Elias LG, Vaz ALL, Favaris AP, Clososki GC, Bento JMS, Nunes TM (2016) Interspecific chemical communication in raids of the robber bee Lestrimelitta limao. Insect Soc 63:339–347. https://doi.org/10.1007/s00040-016-0474-2
Wilson EO (1965) Chemical communication in the social insects. Science 149(3688):1064–1071
Wilson EO (1971) The insect societies. Belknap Press, Harvard University, Cambridge
Wittmann D (1985) Aerial defense of the nest by workers of the stingless bee Trigona (Tetragonisca) angustula (Latreille) (Hymenoptera: Apidae). Behav Ecol Sociobiol 16:111–114
Wittmann D, Radtke R, Zeil J, Lübke G, Francke W (1990) Robber bees (Lestrimelitta limao) and their host chemical and visual cues in nest defense by Trigona (Tetragonisca) angustula (Apidae, Meliponinae). Insect Soc 40:631–641
Yunker WK, Wein DE, Wisenden BD (1999) Conditioned alarm behavior in fathead minnows (Pimephales promelas) resulting from association of chemical alarm pheromone with a nonbiological visual stimulus. J Chem Ecol 25(12):2677–2685
Ziel J, Wittmann D (1989) Visually controlled station-keeping by hovering guard bees of Trigona (Tetragonisca) angustula (Apidae, Meliponinae). J Comp Physiol A 165:711–718
Zufall F, Leinders-Zufall T (2000) The cellular and molecular basis of odor adaptation. Chem Senses 25:473–481
Zufall F, Leinders-Zufall T, Greer CA (2000) Amplification of odor-induced Ca2+ transients by store-operated Ca2+ release and its role in olfactory signal transduction. J Neurophysiol 83:501–512
Acknowledgements
We would like to thank Sonia Villa and Erik Rohner for their support helping to analyze video recordings. We would also like to thank Richard C. Gerkin for input on the analyses, and the anonymous reviewers for their comments on the manuscript.
Funding
C. M. Jernigan was supported by joint grants from Arizona State University and the Smithsonian Tropical Research Institute during data collection, and teaching assistantships from Arizona State University, School of Life Sciences. J. Birgiolas was supported by an Arizona State University Interdisciplinary Graduate Program in Neuroscience fellowship and NIH grants F31DC016811 to JB and R01MH1006674 to Sharon M. Crook. This work was also supported by an award from NIH-NIGMS (GM113967) to BHS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Rueppell
Electronic supplementary material
Figure S1
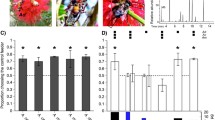
A. Diagram of attack by Tetragonisca angustula (left) on potential robber bee presented to hovering guards (right). B. A photograph of T. anglustula (upper-right), which performed a “death grip” on the wing of a stingless bee raider (left, Trigona sp.). The potential raider was observed near the T. angustula nest and was likely scouting to recruit a raid on the T. angustula nest. (PDF 10789 kb)
Figure S2
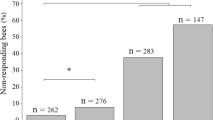
The mean change in guard number from just before (pre-AP) to during synthetic alarm pheromone presentation (AP). There is also an unaccounted change in total guard number from before to after AP presentation, represented by the unaccounted change column. Error bars represent standard error from the mean. (two-tailed, one sample Student’s t test ***p < 0.001) Standing: two-tailed t test, t-value = − 6.81, df = 32, p<<<0.001; hovering: t test, t-value = 6.69, df = 32, p<<<0.001, unaccounted difference: t test, t-value = 3.648, df = 32, p < 0.001). Analysis of a total of 34 observation periods during AP stimulation of 6 colonies. (PDF 597 kb)
Figure S3
A. The mean net inflow of bees before (PR), during (AP) and after (PO) synthetic alarm pheromone presentation pooled by time of day. All morning presentations are denoted in light gray and all afternoon presentations are denoted in dark gray (PR: ANOVA, F-value = 0.103, df = 1, p = 0.05, AP: ANOVA, F-value = 1.01, df = 1, p = 0.32, PO: ANOVA, F-value = 0.004, df = 5, p = 0.948). Error bars represent standard error of the mean. Analysis of a total of 212 observations of 6 colonies, and 3 periods of stimulation before during and after AP. B. The mean percent change in flight activity (measured by frame-to-frame changes in video pixels) from mineral oil (MO) to synthetic alarm pheromone presentation (AP) presentation pooled by time of day presented (ANOVA, F-value = 0.483, df = 1, p = 0.495). Error bars represent standard error of the mean. Analysis of 55 observations of 6 colonies. C. The mean number of guards during mineral oil (MO) presentation pooled by time of day. Column shading as in “A.” No effect of time of day on the number of standing guards (F-value = 1.47, df = 1, p = 0.2330) but a significant effect of time as reported by Grüter et al. (2011) (ANOVA, F-value = 5.84, df = 1, p = 0.02). Error bars represent standard error of the mean (Tukey HSD *p < 0.05). Analysis of 37 observations of 6 colonies during MO stimulation. D. The mean number of guards during synthetic alarm pheromone (AP) presentation pooled by time of day. Column shading as in “A.” During AP there was a significant effect of time of day on both the number of standing (ANOVA, F-value = 6.158, df = 1, p = 0.0180) and hovering guards (ANOVA, F-value = 5.494, df = 1, p = 0.015). Error bars represent standard error of the mean (Tukey HSD *p < 0.05). Analysis of 37 observations of 6 colonies during AP stimulation. (PDF 1353 kb)
Figure S4
The mean net influx rate of bees entering the nest (bees/min) during various stimulus presentations and pairings: sponge only present (SO), sponge with mineral oil stimulus (MO), sponge with synthetic alarm pheromone and Octane pairing (AP/Oct), sponge with octane only (Oct), sponge with synthetic alarm pheromone and 3-Heptanal pairing (AP/3-Hept), and sponge with 3-Heptanal only (3-Hept). First exposure to stimulus is denoted in dark gray and the pooled following stimulus presentations are denoted in light gray. Error bars represent standard error of the mean. We are only presenting the net influx rates as those seemed to be the some of the most robust measures we tested to determine significant impacts on colony behavior. Summary of 163 observations of 5 colonies. Supplemental Analyses: GLM: We performed a model fit of the data that describes a decay and recovery phase defined by the following equation: y = c*(1 + (-TauFast*t)-(TauSlow*t)) (c, TauFast, and TauSlow are fit constants, t is time since previous exposure (hrs), and y is one of the colony measures discussed). This gave us a saddle or inflection point for each measure y. We then performed a GLM analysis separately on the decay phase and recovery phase of the model using R (R core team, 2016) looking at the interacting factors of time since previous exposure (t) and total number of alarm pheromone exposures on the measure y. We used a Gaussian distribution or Poisson distribution during these analyses when appropriate based upon the observed data structure of y. We also performed a chi squared model fit test comparing the above model with a null model in which just number of alarm pheromone (AP) exposures was predicting the measure y. (PDF 703 kb)
Figure S5
Plots of measures during alarm pheromone (AP) exposure minus the measure during mineral oil exposure vs. the time (t) since previous AP exposure (hrs). The points in each plot are the data and the solid line is a model fit of the data, described in supplemental analyses. The vertical dashed line indicates the saddle or inflection point as defined by the model fit, and separates the decay phase and the recovery phases observed in the data. A. The number of attacks during AP stimulation, this is also the difference between this measure and mineral oil, as there were never any attacks observed during mineral oil stimulation. The model fits the saddle point to be 4.5 h. The decay phase shows a non-significant effect of t (GLM, estimate = 0.535, z-value = 1.704, p = 0.089), a significant effect of the number of AP exposures (GLM, estimate = − 1.539, z-value = − 3.766, p < 0.001), and a non-significant interaction (GLM, estimate = − 0.032, z-value = − 0.201, p = 0.841). The decay model still has a better fit of the data than the null (χ2, p = 0.02). The recovery phase shows a non-significant effect of t (GLM, estimate = − 0.01, z-value = − 0.835, p = 0.404), a significant effect of number of AP exposures (GLM, estimate = − 0.662, z-value = − 4.483, p < 0.001), and a significant interaction (GLM, estimate = 0.009, z-value = 2.527, p = 0.011). The recovery model fits significantly better than the null (χ2, p < 0.001). B. The number of bees attacking during AP stimulation, this is also the difference between this measure and mineral oil, as there were never any attacks observed during mineral oil stimulation. The model fits the saddle to be 1.2 h. The decay phase shows a significant effect of t (GLM, estimate = − 1.162, z-value = − 2.853, p = 0.004). There were not enough measures in the 1.2 h to test the number of exposures or the interaction. The recovery phase shows a significant effect of t (GLM, estimate = 0.028, z-value = 2.245, p = 0.025), a non-significant effect of number of AP exposures (GLM, estimate = − 0.242, z-value = − 1.609, p = 0.12), and a non-significant interaction (GLM, estimate = − 0.001, z-value = − 0.533, p = 0.594). The recovery model is significantly better fit than the null (χ2, p < 0.001). C. The net forager influx rate (bees/min) difference between AP and mineral oil stimulation plotted against the time since previous alarm pheromone exposure. The model fits the saddle to be at 3.7 h. The decay phase shows non-significant effect of both t (GLM, estimate = 5.27, t-value = 0.399, p = 0.701) and number of AP exposures on observed measures (GLM, estimate = − 0.466, t-value = − 0.03, p = 0.977), and no difference from null model (χ2, p = 0.9). However, the recovery phase shows a significant effect of t (GLM, estimate = 1.159, t-value = 3.426, p = 0.003), number of exposures (GLM, estimate = 7.935, t-value = 2.198, p = 0.04), and an interaction (GLM, estimate = − 0.283, t-value = − 3.204, p = 0.005). The recovery has a significantly better fit than the null model (χ2, p = 0.003). D. The colony activity change during AP simulation and mineral oil stimulation vs. the time since previous AP exposure (hrs). The model fits the saddle point to be 30.6 h. Both the recovery and the decay showed non-significant differences from the null models (χ2, p > 0.6) and no significant effect of either t or number of AP exposures on activity (GLM, p > 0.5). There was also no significant interaction between the two (GLM, p > 0.6) (PDF 1641 kb)
Table S1
The test statistics for ANOVA and paired t test outputs of the novel odor-alarm pheromone paring tests. Presenting for number of bees attacking, number of attacks, and average percent flight activity change between mineral oil and alarm stimulation. All tests are non-significant. (MP4 356,640 kb) (PDF 1089 kb)
Rights and permissions
About this article
Cite this article
Jernigan, C.M., Birgiolas, J., McHugh, C. et al. Colony-level non-associative plasticity of alarm responses in the stingless honey bee, Tetragonisca angustula. Behav Ecol Sociobiol 72, 58 (2018). https://doi.org/10.1007/s00265-018-2471-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-018-2471-0