Abstract
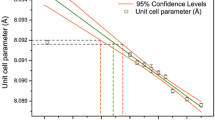
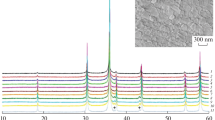
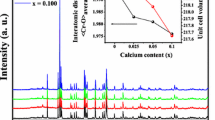
The evolution of the structural environment of \(\hbox {Cr}{^{3+}}\) along the solid solution \(\hbox {ZnAl}_{2-x}\hbox {Cr}_{x}\hbox {O}_4\) has been investigated using a multi-analytical approach. X-ray diffraction confirms that the system follows Vegard’s law. Diffuse reflectance spectra show a decrease of the crystal field parameter with the Cr content, usually related to the increase of the Cr–O bond length in a point charge model. This interpretation is discussed and compared to the data obtained by first-principle calculations based on density functional theory. X-ray absorption near edge structure spectra at the Cr K-edge show a pronounced evolution in the pre-edge with the Cr content, characterised by the appearance of a third feature. Calculations enable to assign the origin of this feature to Cr neighbours. The colour change from pink to brownish pink and eventually green along the solid solution has also been quantified by calculating the L*, a*, b* and x, y coefficients in the system defined by the International Commission on Illumination.










Similar content being viewed by others
References
Ardit M, Cruciani G, Dondi M (2012) Structural relaxation in tetrahedrally coordinated Co\({^{2+}}\) along the gahnite-Co-aluminate spinel solid solution. Am Mineral 97(8–9):1394–1401
Ardit M, Dondi M, Cruciani G (2014) On the structural relaxation around \(\text{ Cr }{^{3+}}\) along binary solid solutions. Eur J Mineral 26(3):359–370
Balan E, De Villiers JPR, Griet Eeckhout S, Glatzel P, Toplis MJ, Fritsch E, Allard T, Galoisy L, Calas G (2006) The oxidation state of vanadium in titanomagnetite from layered basic intrusions. Am Mineral 91(5–6):953–956
Barnes SJ, Roeder P (2001) The range of spinel compositions in terrestrial mafic and ultramafic rocks. J Petrol 42(12):2279–2302
Biagioni C, Pasero M (2014) The systematics of the spinel-type minerals: an overview. Am Mineral 99(7):1254–1264
Bordage A, Rossano S, Horn AH, Fuchs Y (2012) Site partitioning of \(\text{ Cr }{^{3+}}\) in the trichroic alexandrite BeAl\(_2\text{ O }_4\):\(\text{ Cr }{^{3+}}\) crystal: contribution from X-ray absorption spectroscopy. J Phys Condens Matter 24(22):225, 401
Bosi F, Andreozzi GB, Halenius U, Skogby H (2011) Zn-O tetrahedral bond length variations in normal spinel oxides. Am Mineral 96(4):594–598
Brigida C, Poli S, Valle M (2007) High-temperature phase relations and topological constraints in the quaternary system MgO-\(\text{ Al }_2\text{ O }{_3}\)-SiO\({_2}\)-Cr\(_2\text{ O }{_3}\): an experimental study. Am Mineral 92(5–6):735–747
Burns RG (1993) Mineralogical applications of crystal field theory, vol 5. Cambridge University Press, Cambridge
Cabaret D, Bordage A, Juhin A, Arfaoui M, Gaudry E (2010) First-principles calculations of X-ray absorption spectra at the K-edge of 3d transition metals: an electronic structure analysis of the pre-edge. PCCP 12(21):5619–5633
Chan K, Sau J, Zhang P, Cohen M (2007) Ab Initio calculations of phonon splitting in antiferromagnetic ZnCr\(_2\text{ O }_4\). Phys Rev B 75(5):054,304
Evans BW, Frost B (1975) Chrome-spinel in progressive metamorphisma preliminary analysis. Geochimica et Cosmochimica Acta 39(6–7):959–972
Farges F (2009) Chromium speciation in oxide-type compounds: application to minerals, gems, aqueous solutions and silicate glasses. Phys Chem Minerals 36(8):463–481
Fernández-Osorio A, Pineda-Villanueva E, Chávez-Fernández J (2012) Synthesis of nanosized (Zn1xCox)Al2O4 spinels: new pink ceramic pigments. Mater Res Bull 47(2):445–452
Galoisy L (1996) Local versus average structure around cations in minerals from spectroscopic and diffraction measurements. Phys Chem Minerals 23:217
García-Lastra J, Barriuso M, Aramburu J, Moreno M (2005) Origin of the different color of ruby and emerald. Phys Rev B 72(11):113,104
García-Lastra J, Aramburu J, Barriuso M, Moreno M (2006) Optical properties of \(\text{ Cr }{^{3+}}\)-doped oxides: different behavior of two centers in alexandrite. Phys Rev B 74(11):115,118
Gaudry E, Kiratisin A, Sainctavit P, Brouder C, Mauri F, Ramos A, Rogalev A, Goulon J (2003) Structural and electronic relaxations around substitutional \(\text{ Cr }{^{3+}}\) and Fe\({^{3+}}\) ions in corundum. Phys Rev B 67(9):094,108
Gaudry E, Sainctavit P, Juillot F, Bondioli F, Ohresser P, Letard I (2005) From the green color of eskolaite to the red color of ruby: an X-ray absorption spectroscopy study. Phys Chem Minerals 32(10):710–720
Giannozzi P, Baroni S, Bonini N, Calandra M, Car R, Cavazzoni C, Ceresoli D, Chiarotti GL, Cococcioni M, Dabo I, Dal Corso A, de Gironcoli S, Fabris S, Fratesi G, Gebauer R, Gerstmann U, Gougoussis C, Kokalj A, Lazzeri M, Martin-Samos L, Marzari N, Mauri F, Mazzarello R, Paolini S, Pasquarello A, Paulatto L, Sbraccia C, Scandolo S, Sclauzero G, Seitsonen AP, Smogunov A, Umari P, Wentzcovitch RM (2009) QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J Phys Condens Matter 21(39):395,502
Gougoussis C, Calandra M, Seitsonen A, Mauri F (2009) First-principles calculations of X-ray absorption in a scheme based on ultrasoft pseudopotentials: from \(\alpha\)-quartz to high-Tc compounds. Phys Rev B 80(7):075,102
Halenius U, Andreozzi GB, Skogby H (2010) Structural relaxation around \(\text{ Cr }{^{3+}}\) and the red-green color change in the spinel (sensu stricto)-magnesiochromite (MgAl\(_2\text{ O }_4\)-MgCr\(_2\text{ O }_4\)) and gahnite-zincochromite (ZnAl\(_2\text{ O }_4\)-ZnCr\(_2\text{ O }_4\)) solid-solution series. Am Mineral 95(4):456–462
Haskel D (1999) http://www.aps.anl.gov/~haskel/fluo.html
Ikeda K, Nakamura Y, Masumoto K, Shima H (1997) Optical spectra of synthetic spinels in the system MgAl\(_2\text{ O }_4\)-MgCr\(_2\text{ O }_4\). J Am Ceram Soc 80(10):2672–2676
Irvine TN (1965) Chromian spinel as a petrogenetic indicator: part 1. Theory. Can J Earth Sci 2(6):648–672
Irvine TN (1967) Chromian spinel as a petrogenetic indicator: part 2. Petrologic applications. Can J Earth Sci 4(1):71–103
Juhin A (2008) Propriétés électroniques et structurales du chrome en impureté dans les cristaux. Approche expérimentale et théorique. Sciences des matériaux. Paris, Paris 6. PhD thesis, thèse de doctorat
Juhin A, Calas G, Cabaret D, Galoisy L, Hazemann JL (2007) Structural relaxation around substitutional \(\text{ Cr }{^{3+}}\) in MgAl\(_2\text{ O }_4\). Phys Rev B 76(5):054,105
Juhin A, Brouder C, Arrio MA, Cabaret D, Sainctavit P, Balan E, Bordage A, Seitsonen A, Calas G, Eeckhout S, Glatzel P (2008a) X-ray linear dichroism in cubic compounds: the case of \(\text{ Cr }{^{3+}}\) in MgAl\(_2\text{ O }_4\). Phys Rev B 78(19):195,103
Juhin A, Calas G, Cabaret D, Galoisy L, Hazemann JL (2008b) Structural relaxation around substitutional \(\text{ Cr }{^{3+}}\) in pyrope garnet. Am Mineral 93(5–6):800–805
Kashii N, Maekawa H, Hinatsu Y (1999) Dynamics of the Cation Mixing of MgAl\(_2\text{ O }_4\) and ZnAl\(_2\text{ O }_4\) Spinel. J Am Ceram Soc 82(7):1844–1848
Klemme S (2004) The influence of Cr on the garnetspinel transition in the Earth’s mantle: experiments in the system MgOCr\(_2\text{ O }_3\)SiO\(_2\) and thermodynamic modelling. Lithos 77(1–4):639–646
König E, Kremer S (1977) Ligand field energy diagrams. Plenum Publishing Corporation, New York
Kubelka P, Munk F (1931) Ein Beitrag zur Optik der Farbanstriche. Z Tech Phys 12(11a):593–601
Lavina B, Salviulo G, Giusta AD (2002) Cation distribution and structure modelling of spinel solid solutions. Phys Chem Minerals 29(1):10–18
Lenaz D, Princivalle F (2005) The crystal chemistry of detrital chromian spinel from the southeastern alps and outer dinarides: the discrimination of supplies from areas of similar tectonic setting? Can Mineral 43(4):1305–1314
Malézieux JM, Piriou B (1988) Relation entre la composition chimique et le comportement vibrationnel de spinelles de synthèse et de chromites naturelles en microspectrométrie Raman. Bulletin de minéralogie 111(6):649–669
Marfunin AS, Egorova NG, Mishchenko AG (1979) Physics of minerals and inorganic materials: an introduction. Springer, Berlin
Martins J, Zunger A (1984) Bond lengths around isovalent impurities and in semiconductor solid solutions. Phys Rev B 30(10):6217–6220
Martos M, Martínez M, Cordoncillo E, Escribano P (2007) Towards more ecological ceramic pigments: study of the influence of glass composition on the colour stability of a pink chromium-doped ceramic pigment. J Eur Ceram Soc 27(16):4561–4567
O’Neill HSC, Dollase WA (1994) Crystal structures and cation distributions in simple spinels from powder XRD structural refinements: MgCr\(_2\text{ O }_4\), ZnCr\(_2\text{ O }_4\), Fe\(_3\text{ O }_4\) and the temperature dependence of the cation distribution in ZnAl\(_2\text{ O }_4\). Phys Chem Minerals 20(8):541–555
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77(18):3865–3868
Perinelli C, Bosi F, Andreozzi GB, Conte AM, Armienti P (2014) Geothermometric study of Cr-spinels of peridotite mantle xenoliths from northern Victoria Land (Antarctica). Am Mineral 99(4):839–846
Prim S, García A, Galindo R, Cerro S, Llusar M, Folgueras M, Monrós G (2013) Pink ceramic pigments based on chromium doped M(\(\text{ Al }_{2-x}\text{ Cr }_x\))O\(_4\), M = Mg, Zn, normal spinel. Ceram Int 39(6):6981–6989
Rietveld HM (1969) A profile refinement method for nuclear and magnetic structures. J Appl Crystallography 2(2):65–71
Rodríguez-Carvajal J (1993) Recent advances in magnetic structure determination by neutron powder diffraction. Phys B Condens Matter 192(1–2):55–69
Solé VA, Papillon E (2004) PyMCA: X-Ray Spectra Visualization and Analysis in Python. In: NOBUGS 2004 conference, Paul Scherrer Institute, Villigen PSI, Switzerland
Taillefumier M, Cabaret D, Flank AM, Mauri F (2002) X-ray absorption near-edge structure calculations with the pseudopotentials: application to the K edge in diamond and \(\alpha\)-quartz. Phys Rev B 66(19):195,107
Wood DL (1968) Optical spectrum of \(\text{ Cr }{^{3+}}\) ions in spinels. J Chem Phys 48(11):5255
Wyszecki G, Stiles WS (2008) Color science: concepts and methods, quantitative data and formulae. Wiley, Chichester
Acknowledgments
This work was supported by the Réseau Francilien sur les oxydes fonctionnels (DIM Oxymore) and the Région Ile-de-France. We acknowledge the European Synchrotron Radiation Facility for provision of synchrotron radiation facilities at beamline ID21, through the proposal HG43. The calculations were partly performed using HPC resources from GENCI-IDRIS (100172-2015). L.V. acknowledges M. Chassé for his help with the chromaticity parameters and fruitful discussions. E.R. gratefully acknowledges the support of the Postdoctoral Fellowship of the Hungarian Academy of Sciences, the European Research Council (ERC Starting Grant No. 259709), and the French-Hungarian Balaton-TéT bilateral research program (Project No. TET_11_FR-XTHEOEXP) as well.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Verger, L., Dargaud, O., Rousse, G. et al. Spectroscopic properties of \(\hbox {Cr}^{3+}\) in the spinel solid solution \(\hbox {ZnAl}_{2-x}\hbox {Cr}_{x}\hbox {O}_4\) . Phys Chem Minerals 43, 33–42 (2016). https://doi.org/10.1007/s00269-015-0771-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-015-0771-8