Abstract
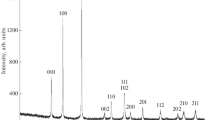
Single crystals of B2O3 are needed for the precise determination of the refractive indices used to calculate the electronic polarizability α of 3-coordinated boron. The α(B) values in turn are used to predict mean refractive indices of borate minerals. Since the contribution of boron to the total polarizability of a mineral is very low, the synthetic compound B2O3 represents an ideal model system because of its high molar content of boron. Millimeter-sized crystals were synthesized at 1 GPa in a piston-cylinder apparatus. The samples were heated above the liquidus (800 °C), subsequently cooled at 15 °C/h to 500 °C and finally quenched. The refractive indices were determined by the immersion method using a microrefractometer spindle stage. The refractive indices n o = 1.653 (3) and n e = 1.632 (3) correspond to a total polarizability for B2O3 of α = 4.877 Å3. These values were used to determine the electronic polarizability of boron of α(B) = 0.16 Å3. Although the surface of the B2O3 crystals was coated with a hydrous film immediately after being exposed to air, its bulk crystallinity is retained for a period of at least 2 months.




Similar content being viewed by others
References
Aziz MJ, Nygren E, Hays JF, Turnbull D (1985) Crystal growth kinetics of boron oxide under pressure. J Appl Phys 57:2233–2242
Berger SV (1952) The crystal structure of B2O3. Acta Crystallogr 5:389
Berger SV (1953) The crystal structure of boron oxide. Acta Chem Scand 7:611–622
Bloss FD (1981) The spindle stage: principles and practice. Cambridge University Press, Cambridge
Bloss FD, Gunter M, Su S-C, Wolfe HE (1983) Gladstone–Dale constants: a new approach. Can Mineral 21:93–99
Cole SS, Taylor NW (1935) The system Na2O–B2O3, I Preparation of crystalline B2O3 and some of its physical properties. J Am Ceram Soc 18:55–58
Effenberger H, Lengauer CL, Parthé E (2001) Trigonal B2O3 with higher space-group symmetry: results of a reevaluation. Monatsh Chem 132:1515–1517
Eggleton RA (1991) Gladstone–Dale constants for the major elements in silicates: coordination number, polarizability, and the Lorentz-Lorentz relation. Can Mineral 29:525–532
Fischer RX (1985) STRUPLO84, a Fortran plot program for crystal structure illustrations in polyhedral representation. J Appl Crystallogr 18:258–262
Fischer RX, Messner T (2013) STRUPLO, a new version of the structure drawing program. Fachbereich Geowissenschaften. Universität Bremen. www.brass.uni-bremen.de
Fischer RX, Tillmanns E (1988) The equivalent isotropic displacement factor. Acta Crystallogr C 44:775–776
Gunter ME, Downs RT, Bartelmehs KL, Evans SH, Pommier CJS, Grow JS, Sanchez MS, Bloss FD (2005) Optic properties of centimeter-sized crystals determined in air with the spindle stage using EXCALIBRW. Am Mineral 90:1648–1654
Gurr GE, Montgomery PW, Knutson CD, Gorres BT (1970) The crystal structure of trigonal diboron trioxide. Acta Crystallogr B 26:906–915
Islam MM, Bredow T, Minot C (2006) Comparison of trigonal B2O3 structures with high and low space-group symmetry. Chem Phys Lett 418:565–568
Kracek FC, Morey GW, Merwin HE (1938) The system water-boron oxide. Am J Sci 35A:143–171
MacKenzie JD, Claussen WF (1961) Crystallization and phase relations of boron trioxide at high pressures. J Am Ceram Soc 44:79–81
Mandarino JA (1976) The Gladstone–Dale relationship. Part I: derivation of new constants. Can Mineral 16:169–174
Mandarino JA (1979) The Gladstone–Dale relationship. Part III: some general applications. Can Mineral 17:71–76
Mandarino JA (1981) The Gladstone–Dale relationship. Part IV: the compatibility concept and its application. Can Mineral 19:441–450
Mandarino JA (2007) The Gladstone–Dale compatibility of minerals and its use in selecting mineral species for further study. Can Mineral 45:1307–1324
Medenbach O (1985) A new microrefractometer spindle-stage and its application. Fortschr Mineral 63:111–133
Nickel EH, Grice JD (1998) The IMA Commission on New Minerals and Mineral Names: procedures and guidelines on mineral nomenclature, 1998. Can Mineral 36:913–926
Nieto-Sanz D, Loubeyre P, Crichton W, Mezouar M (2004) X-ray study of the synthesis of boron oxides at high pressure: phase diagram and equation of state. Phys Rev B 70(214108):1–6
Prewitt CT, Shannon RD (1968) Crystal structure of a high-pressure form of B203. Acta Crystallogr B 24:869–874
Qin F, Li RK (2011) Predicting refractive indices of the borate optical crystals. J Cryst Growth 318:642–644
Shannon RD, Fischer RX (2016) Empirical electronic polarizabilities of ions for the determination of refractive indices. I. Oxides and oxysalts. Am Mineral (in press)
Sheldrick GM (1997) SHELXL-97, a program for crystal structure refinement, Release 97-2. University of Goettingen, Göttingen
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A 64:112–122
Strong SL, Kaplow R (1968) The structure of crystalline B2O3. Acta Crystallogr B 24:1032–1036
Strong SL, Wells AF, Kaplow R (1971) On the crystal structure of B2O3. Acta Crystallogr B 27:1662–1663
Uhlmann DR, Hays JF, Turnbull D (1967) Effect of high pressure on B2O3: crystallisation, densification, and the crystallisation anomaly. Phys Chem Glasses 8:1–10
Acknowledgments
We thank the Deutsche Forschungsgemeinschaft (DFG) for funding this project under grant Fi442/21-1.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Burianek, M., Birkenstock, J., Mair, P. et al. High-pressure synthesis, long-term stability of single crystals of diboron trioxide, B2O3, and an empirical electronic polarizability of [3]B3+ . Phys Chem Minerals 43, 527–534 (2016). https://doi.org/10.1007/s00269-016-0813-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-016-0813-x