Abstract
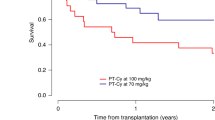
Cytokine release syndrome (CRS), occurring in more than 70% of HLA-haploidentical hematopoietic stem-cell transplantations with post-transplant cyclophosphamide (PT/CY-haplo), can lead to hemodynamic instability and worsen clinical outcomes. A calcineurin inhibitor is initiated after cyclophosphamide administration in the commonly used PT/CY regimens. Here, we conducted a phase I/II, prospective, single-center trial of PT/CY-haplo to evaluate the safety and efficacy of cyclophosphamide on days 3 and 5 along with cyclosporin and mycophenolate mofetil started from day − 1. Thirty-five adults with hematologic malignancies were enrolled. Myeloablative and reduced-intensity conditioning were used in 25 and 10 patients, respectively. Graft sources were bone marrow in 11 patients and mobilized peripheral blood stem cells in 24 patients. Disease-free survival on day 100, the primary endpoint, was 86% (95% confidence interval (CI), 69–94), which was over the predefined threshold of 50%. Unexpectedly, only 20% (95% CI, 8.4–37) of patients developed fever of > 38 °C early after graft infusion, all CRS grade 1, and all of which resolved just after cyclophosphamide administration. The cumulative incidences of grades II–IV acute graft-versus-host disease (GVHD), III–IV acute GVHD, and moderate-severe chronic GVHD were 23% (95% CI, 11–38), 6% (95% CI, 1–17), and 11% (95% CI, 4–25), respectively. The 3-year overall survival rate was 49% (95% CI, 31–64). Our results suggest that administration of cyclosporine and mycophenolate mofetil prior to PT/CY can reduce the frequency and severity of CRS without increasing GVHD. UMIN Clinical Trial Registry numbers: 000006631 and 000015694




Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL (2014) Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124:188–195. https://doi.org/10.1182/blood-2014-05-552729
Arango M, Combariza JF (2017) Fever after peripheral blood stem cell infusion in haploidentical transplantation with post-transplant cyclophosphamide. Hematol Oncol Stem Cell Ther 10:79–84. https://doi.org/10.1016/j.hemonc.2017.03.001
Sugita J, Kagaya Y, Miyamoto T, Shibasaki Y, Nagafuji K, Ota S, Furukawa T, Nara M, Akashi K, Taniguchi S, Harada M, Matsuo K, Teshima T (2019) Myeloablative and reduced-intensity conditioning in HLA-haploidentical peripheral blood stem cell transplantation using post-transplant cyclophosphamide. Bone Marrow Transplant 54:432–441. https://doi.org/10.1038/s41409-018-0279-1
Solh MM, Dickhaus E, Solomon SR, Morris LE, Zhang X, Holland HK, Bashey A (2019) Fevers post infusion of T cell replete hla mismatched haploidentical hematopoietic stem cells with post-transplant cyclophosphamide: risk factors and impact on transplant outcomes. Bone Marrow Transplant 54:1756–1763. https://doi.org/10.1038/s41409-019-0522-4
Abboud R, Keller J, Slade M, DiPersio JF, Westervelt P, Rettig MP, Meier S, Fehniger TA, Abboud CN, Uy GL, Vij R, Trinkaus KM, Schroeder MA, Romee R (2016) Severe cytokine-release syndrome after T cell-replete peripheral blood haploidentical donor transplantation is associated with poor survival and anti-IL-6 therapy is safe and well tolerated. Biol Blood Marrow Transplant 22:1851–1860. https://doi.org/10.1016/j.bbmt.2016.06.010
Imus PH, Blackford AL, Bettinotti M, Luznik L, Fuchs EJ, Huff CA, Gladstone DE, Ambinder RF, Borrello IM, Fuchs RJ, Swinnen LJ, Wagner-Johnston N, Gocke CB, Ali SA, Bolaños-Meade FJ, Jones RJ, Dezern AE (2019) Severe cytokine release syndrome after haploidentical peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 25:2431–2437. https://doi.org/10.1016/j.bbmt.2019.07.027
Cho C, Perales MA (2016) Rapid identification of cytokine release syndrome after haploidentical PBSC transplantation and successful therapy with tocilizumab. Bone Marrow Transplant 51:1620–1621. https://doi.org/10.1038/bmt.2016.229
Mariotti J, Taurino D, Marino F, Bramanti S, Sarina B, Morabito L, De Philippis C, Di Vito C, Mavilio D, Carlo-Stella C, Della Porta M, Santoro A, Castagna L (2019) Pretransplant active disease status and HLA class II mismatching are associated with increased incidence and severity of cytokine release syndrome after haploidentical transplantation with posttransplant cyclophosphamide. Cancer Med 9:52–61. https://doi.org/10.1002/cam4.2607
Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, Gooley TA, Piantadosi S, Kaup M, Ambinder RF, Huff CA, Matsui W, Bolaños-Meade J, Borrello I, Powell JD, Harrington E, Warnock S, Flowers M, Brodsky RA, Sandmaier BM, Storb RF, Jones RJ, Fuchs EJ (2008) HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 14:641–650. https://doi.org/10.1016/j.bbmt.2008.03.005
Raiola AM, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F, Bregante S, Van Lint MT, Geroldi S, Luchetti S, Ballerini F, Miglino M, Varaldo R, Bacigalupo A (2013) Unmanipulated haploidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant 19:117–122. https://doi.org/10.1016/j.bbmt.2012.08.014
Bacigalupo A, Maria Raiola A, Dominietto A, Di Grazia C, Gualandi F, Lint MTV, Chiusolo P, Laurenti L, Sora F, Giammarco S, Angelucci E (2019) Graft versus host disease in unmanipulated haploidentical marrow transplantation with a modified post-transplant cyclophosphamide (PT-CY) regimen: an update on 425 patients. Bone Marrow Transplant 54(Suppl 2):708–712. https://doi.org/10.1038/s41409-019-0594-1
Szydlo R, Goldman JM, Klein JP, Gale RP, Ash RC, Bach FH, Bradley BA, Casper JT, Flomenberg N, Gajewski JL, Gluckman E, Henslee-Downey PJ, Hows JM, Jacobsen N, Kolb HJ, Lowenberg B, Masaoka T, Rowlings PA, Sondel PM, van Bekkum DW, van Rood JJ, Vowels MR, Zhang MJ, Horowitz MM (1997) Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol 15:1767–1777. https://doi.org/10.1200/JCO.1997.15.5.1767
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED (1995) 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant 15:825–828
Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, Maziarz RT, Antin JH, Soiffer RJ, Weisdorf DJ, Rizzo JD, Horowitz MM, Saber W (2014) Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood 123:3664–3671. https://doi.org/10.1182/blood-2014-01-552984
Lin DY (1997) Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med 16:901–910. https://doi.org/10.1002/(SICI)1097-0258(19970430)16:8<901::AID-SIM543>3.0.CO;2-M
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458. https://doi.org/10.1038/bmt.2012.244
McCurdy SR, Muth ST, Tsai HL, Symons HJ, Huff CA, Matsui WH, Borrello I, Gladstone DE, Swinnen LJ, Cooke KR, Brodsky RA, Bolaños-Meade J, Ambinder RF, Varadhan R, Luznik L, Jones RJ, Bettinot MP, Fuchs EJ (2018) Early fever after haploidentical bone marrow transplantation correlates with class II HLA-mismatching and myeloablation but not outcomes. Biol Blood Marrow Transplant 24:2056–2064. https://doi.org/10.1016/j.bbmt.2018.06.004
Raj RV, Hamadani M, Szabo A, Pasquini MC, Shah NN, Drobyski WR, Shaw BE, Saber W, Rizzo JD, Jerkins J, Fenske TS, D’Souza A, Dhakal B, Zhang C, Konings S, Hari PN, Chhabra S (2018) Peripheral blood grafts for T cell-replete haploidentical transplantation increase the incidence and severity of cytokine release syndrome. Biol Blood Marrow Transplant 24:1664–1670. https://doi.org/10.1016/j.bbmt.2018.04.010
O’Donnell P, Raj K, Pagliuca A (2015) High fever occurring 4 to 5 days post-transplant of haploidentical bone marrow or peripheral blood stem cells after reduced-intensity conditioning associated with the use of post-transplant cyclophosphamide as prophylaxis for graft-versus-host disease. Biol Blood Marrow Transplant 21:197–198. https://doi.org/10.1016/j.bbmt.2014.10.008
Nishimoto M, Hirose A, Koh H, Nakamae M, Nanno S, Okamura H, Nakane T, Nakashima Y, Hino M, Nakamae H (2019) Clinical impacts of using serum IL-6 level as an indicator of cytokine release syndrome after HLA-haploidentical transplantation with post-transplantation cyclophosphamide. Biol Blood Marrow Transplant 25:2061–2069. https://doi.org/10.1016/j.bbmt.2019.06.003
Chen Y, Huang XJ, Wang Y, Liu KY, Chen H, Chen YH, Zhang XH, Wang FR, Han W, Wang JZ, Yan CH, Zhang YY, Sun YQ, Xu LP (2015) Febrile reaction associated with the infusion of haploidentical peripheral blood stem cells: incidence, clinical features, and risk factors. Transfusion 55:2023–2031. https://doi.org/10.1111/trf.13167
Abid MB, Hamadani M, Szabo A, Hari PN, Graham MB, Frank MO, Collier WS, Abedin S, Jerkins JH, Pasquini MC, Runaas L, Shah NN, Chhabra S (2020) Severity of cytokine release syndrome and its association with infections after T cell-replete haploidentical related donor transplantation. Biol Blood Marrow Transplant. 26:1670–1678. https://doi.org/10.1016/j.bbmt.2020.06.006
Isobe M, Konuma T, Kato S, Tanoue S, Mizusawa M, Oiwa-Monna M, Takahashi S, Tojo A (2019) Development of pre-engraftment syndrome, but not acute graft-versus-host disease, reduces relapse rate of acute myelogenous leukemia after single cord blood transplantation. Biol Blood Marrow Transplant 25:1187–1196. https://doi.org/10.1016/j.bbmt.2019.02.007
Luznik L, Fuchs EJ (2010) High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunol Res 47:65–77. https://doi.org/10.1007/s12026-009-8139-0
Solomon SR, Sizemore CA, Sanacore M, Zhang X, Brown S, Holland HK, Morris LE, Bashey A (2015) Total body irradiation-based myeloablative haploidentical stem cell transplantation is a safe and effective alternative to unrelated donor transplantation in patients without matched sibling donors. Biol Blood Marrow Transplant 21:1299–1307. https://doi.org/10.1016/j.bbmt.2015.03.003
Bashey A, Zhang MJ, McCurdy SR, St Martin A, Argall T, Anasetti C, Ciurea SO, Fasan O, Gaballa S, Hamadani M, Munshi P, Al Malki MM, Nakamura R, O’Donnell PV, Perales MA, Raj K, Romee R, Rowley S, Rocha V, Salit RB, Solh M, Soiffer RJ, Fuchs EJ, Eapen M (2017) Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T cell-replete haploidentical donor transplantation using post-transplant cyclophosphamide. J Clin Oncol 35:3002–3009. https://doi.org/10.1200/JCO.2017.72.8428
Ruggeri A, Labopin M, Bacigalupo A, Gülbas Z, Koc Y, Blaise D, Bruno B, Irrera G, Tischer J, Diez-Martin JL, Castagna L, Ciceri F, Mohty M, Nagler A (2018) Bone marrow versus mobilized peripheral blood stem cells in haploidentical transplants using posttransplantation cyclophosphamide. Cancer 124:1428–1437. https://doi.org/10.1002/cncr.31228
Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M (2018) CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med 24(6):731–738. https://doi.org/10.1038/s41591-018-0041-7
Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, Sanvito F, Ponzoni M, Doglioni C, Cristofori P, Traversari C, Bordignon C, Ciceri F, Ostuni R, Bonini C, Casucci M, Bondanza A (2018) Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med 24(6):739–748. https://doi.org/10.1038/s41591-018-0036-4
Hay KA, Hanafi LA, Li D, Gust J, Liles WC, Wurfel MM, López JA, Chen J, Chung D, Harju-Baker S, Cherian S, Chen X, Riddell SR, Maloney DG, Turtle CJ (2017) Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T cell therapy. Blood 130(21):2295–2306. https://doi.org/10.1182/blood-2017-06-793141
Obstfeld AE, Frey NV, Mansfield K, Lacey SF, June CH, Porter DL, Melenhorst JJ, Wasik MA (2017) Cytokine release syndrome associated with chimeric-antigen receptor T cell therapy: clinicopathological insights. Blood 130(23):2569–2572. https://doi.org/10.1182/blood-2017-08-802413
Acknowledgements
We thank Dr. Bryan J. Mathis (Medical English Communications Center, University of Tsukuba) for grammatical review and advice.
Funding
This work was supported by Japan Agency for Medical Research and Development (AMED), under Grant Number JP15Aek0510005.
Author information
Authors and Affiliations
Contributions
NK designed and conducted the study, interpreted the data, and wrote the manuscript. TS, TK, MK, YY, HN, NO, MS-Y, and YH conducted the study and reviewed the manuscript. SC supervised the project, discussed data analysis, and reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Institutional Review Board of University of Tsukuba Hospital (approval #H23-081 and #H26-184). Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Kurita, N., Sakamoto, T., Kato, T. et al. Early administration of cyclosporine may reduce the incidence of cytokine release syndrome after HLA-haploidentical hematopoietic stem-cell transplantation with post-transplant cyclophosphamide. Ann Hematol 100, 1295–1301 (2021). https://doi.org/10.1007/s00277-021-04439-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-021-04439-6