Abstract
Purpose
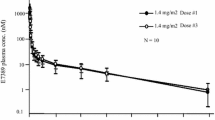
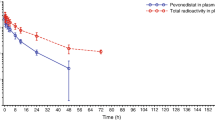
Butyrate is a small polar compound able to produce terminal differentiation and apoptosis in a variety of in vitro models at levels above 50–100 μM. Previously our group demonstrated that daily oral administration of the prodrug, tributyrin, is able to briefly achieve levels >100 μM. Given in vitro data that differentiating activity requires continuous butyrate exposure, the short t1/2 of the drug and a desire to mimic the effects of an intravenous infusion, we evaluated a three times daily schedule.
Patients and methods
Enrolled in this study were 20 patients with advanced solid tumors for whom no other therapy was available, had life expectancy greater than 12 weeks, and normal organ function. They were treated with tributyrin at doses from 150 to 200 mg/kg three times daily. Blood was sampled for pharmacokinetic analysis prior to dosing and at 15 and 30 min and 1, 1.5, 2, 2.5, 3, 3.5 and 4 h thereafter.
Results
The patients entered comprised 15 men and 5 women with a median age of 61 years (range 30–74 years). Prior therapy regimens included: chemotherapy (median two prior regimens, range none to five), radiation therapy (one), no prior therapy (one). There was no dose-limiting toxicity. Escalation was halted at the 200 mg/kg three times daily level due to the number of capsules required. A median butyrate concentration of 52 μM was obtained but there was considerable interpatient variability. No objective responses were seen. There were four patients with prolonged disease stabilization ranging from 3 to 23 months; median progression-free survival was 55 days. Two patients with chemotherapy-refractory non-small-cell lung cancer had survived for >1 year at the time of this report without evidence of progression.
Conclusion
Tributyrin is well tolerated and levels associated with in vitro activity are achieved with three times daily dosing.

Similar content being viewed by others
References
Prasad K (1980) Butyric acid: a small fatty acid with diverse biologic functions. Life Sci 27:1351–1358
Pellizzaro C, Coradini D, Daniotti A, Abolafio G, Daidone MG (2001) Modulation of cell cycle-related protein expression by sodium butyrate in human non-small cell lung cancer cell lines. Int J Cancer 91:654–657
Davis T, Kennedy C, Chiew YE, Clarke CL, deFazio A (2000) Histone deacetylase inhibitors decrease proliferation and modulate cell cycle gene expression in normal mammary epithelial cells. Clin Cancer Res 6:4334–4342
Wang QM, Feinman R, Kashanchi F, Houghton JM, Studzinski GP, Harrison LE (2000) Changes in E2F binding after phenylbutyrate-induced differentiation of Caco-2 colon cancer cells. Clin Cancer Res 6:2951–2958
Giermasz A, Nowis D, Jalili A, et al (2001) Antitumor activity of tributyrin in murine melanoma model. Cancer Lett 64:143–148
Maier, Reich E, Martin R, et al (2000) Tributyrin induces differentiation, growth arrest and apoptosis in androgen sensitive and androgen-resistant human prostate cancer cell lines. Int J Cancer 88:245–251
Conley BA, Egorin MJ, Tait N, et al (1998) Phase I study of the orally administered butyrate prodrug, tributyrin, in patients with solid tumors. Clinical Cancer Res 4:629–634
Gibaldi M, Perrier D (1982) Pharmacokinetics, 2nd edn. Marcel Dekker, New York
Warrell RP, He L-Z, Richon V, Calleja E, Pandolfi PP (1998) Therapeutic targeting of transcription in acute promyelocytic leukemia by use of an inhibitor of histone deacetylase. J Natl Cancer Inst 90:1621–1625
Newmark HL, Lupton JR, Young CW (1994) Butyrate as a differentiating agent: pharmacokinetics, analogues and current status. Cancer Lett 78:1–5
Chen Z-X, Breitman T (1994) Tributyrin: a prodrug of butyric acid for potential clinical application in differentiation therapy. Cancer Res 54:3494–3499
Perrine SP, Ginger GD, Faller DV, et al (1993) A short term trial of butyrate to stimulate fetal-globin-gene expression in the beta-globin disorders. N Engl J Med 328:81–86
Carducci M, Bowling MK, Eisenberger M, et al. (1987) Phenylbutyrate (PB) for refractory solid tumors: phase I clinical and pharmacologic evaluation of intravenous and oral PB. Anticancer Res 17:3972–3973
Parodi PW (1997) Cows' milk fat components as potential anticarcinogenic agents. J Nutr 127:1055–1060
Marks PA, Richon VM, Rifkind RA (2001) Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst 92:1210–1216
Witt O, Schmejkal S, Pekrun A (2000) Tributyrin plus all-trans-retinoic acid efficiently induces fetal hemoglobin expression in human erythroleukemia cells. Am J Hematol 64:319–321
Schroder CP, Maurer HR (2001) Tributyrin enhances the cytotoxic activity of interleukin2/interleukin 12-stimulated human natural killer cells against LS 174T colon cancer cells in vitro. Cancer Immunol Immunother 50:69–76
Siu LL, Von Hoff DD, Rephaeli A, et al (1998) Activity of pivaoyloxymethyl butyrate, a novel anticancer agent, on primary human tumor colony forming units. Invest New Drugs 16:113–119
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by U01 CA 69854, awarded by the Division of Cancer Treatment, National Cancer Institute, NIH, Bethesda, MD 20892, and was partially presented at the 37th Annual Meeting of the American Society of Clinical Oncology.
Rights and permissions
About this article
Cite this article
Edelman, M.J., Bauer, K., Khanwani, S. et al. Clinical and pharmacologic study of tributyrin: an oral butyrate prodrug. Cancer Chemother Pharmacol 51, 439–444 (2003). https://doi.org/10.1007/s00280-003-0580-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-003-0580-5