Abstract
Purpose
Combination therapy has generated a significant interest in the clinical setting since certain agents, with known mechanisms of action and non-overlapping toxicities may increase the therapeutic potential of anticancer drugs by decreasing systemic toxicity and overcoming drug resistance. Doxorubicin and docetaxel, two standard antineoplastic agents in hormone-refractory prostate cancer (HRPC) therapy and ciprofloxacin were evaluated singly and in several simultaneous and sequential drug combination schemes, against PC-3 and LNCaP cell lines.
Methods
Cellular viability was determined by the resazurin assay and the assessment of synergism, additivity or antagonism was carried out by the median effect analysis. The importance of dose, exposure time and type of administration were investigated and compared.
Results
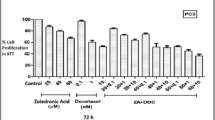
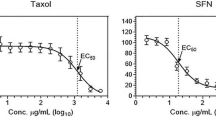
Ciprofloxacin–doxorubicin or docetaxel combinations resulted in prominent additive or synergistic effects in both cell lines, when the cells were pre-treated with ciprofloxacin. These results suggest a rationale for dose reduction of doxorubicin and docetaxel in prostate cancer therapy, since the doses needed to achieve 50% cell death may be decreased by approximately 4- to 15-fold or 3- to 8-fold, respectively, after a pre-treatment with ciprofloxacin. In contrast, the referred combinations yielded moderate antagonistic effects when used concurrently in this in vitro system.
Conclusions
Ciprofloxacin sensitized HRPC cells to doxorubicin or docetaxel-induced growth inhibition and, therefore, may play a role as chemosensitizing agent in prostate cancer treatment.





Similar content being viewed by others
References
Anderson VE, Zaniewski RP, Kaczmarek FS, Gootz TD, Osheroff N (1999) Quinolones inhibit DNA religation mediated by Staphylococcus aureus topoisomerase IV. Changes in drug mechanism across evolutionary boundaries. J Biol Chem 274(50):35927–35932
Aranha O, Grignon R, Fernandes N, McDonnell TJ, Wood DP Jr, Sarkar FH (2003) Suppression of human prostate cancer cell growth by ciprofloxacin is associated with cell cycle arrest and apoptosis. Int J Oncol 22(4):787–794
Aranha O, Wood DP Jr, Sarkar FH (2000) Ciprofloxacin mediated cell growth inhibition, S/G2-M cell cycle arrest, and apoptosis in a human transitional cell carcinoma of the bladder cell line. Clin Cancer Res 6(3):891–900
Bakshi RP, Galande S, Muniyappa K (2001) Functional and regulatory characteristics of eukaryotic type II DNA topoisomerase. Crit Rev Biochem Mol Biol 36(1):1–37
Boland MP, Fitzgerald KA, O’Neill LA (2000) Topoisomerase II is required for mitoxantrone to signal nuclear factor kappa B activation in HL60 cells. J Biol Chem 275(33):25231–25238
Budman DR, Calabro A, Kreis W (2002) Synergistic and antagonistic combinations of drugs in human prostate cancer cell lines in vitro. Anticancer Drugs 13(10):1011–1016
Capranico G, Binaschi M (1998) DNA sequence selectivity of topoisomerases and topoisomerase poisons. Biochim Biophys Acta 1400(1–3):185–194
Chou TC (1994) Assessment of synergistic and antagonistic effects of chemotherapeutic agents in vitro. Contrib Gynecol Obstet 19:91–107
Chou TC, Talalay P (1983) Analysis of combined drug effects: a new look at a very old problem. Trends pharmacol Sci 4:450–454
Chou TC, Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55
Chou TC, Tan QH, Sirotnak FM (1993) Quantitation of the synergistic interaction of edatrexate and cisplatin in vitro. Cancer Chemother Pharmacol 31(4):259–264
Di Lorenzo G, Autorino R, De Laurentiis M, Bianco R, Lauria R, Giordano A, De Sio M, D’Armiento M, Bianco AR, De Placido S (2003) Is there a standard chemotherapeutic regimen for hormone-refractory prostate cancer? Present and future approaches in the management of the disease. Tumori 89(4):349–360
El-Rayes BF, Grignon R, Aslam N, Aranha O, Sarkar FH (2002) Ciprofloxacin inhibits cell growth and synergises the effect of etoposide in hormone resistant prostate cancer cells. Int J Oncol 21(1):207–211
Elsea SH, McGuirk PR, Gootz TD, Moynihan M, Osheroff N (1993) Drug features that contribute to the activity of quinolones against mammalian topoisomerase II and cultured cells: correlation between enhancement of enzyme-mediated DNA cleavage in vitro and cytotoxic potential. Antimicrob Agents Chemother 37(10):2179–2186
Elsea SH, Westergaard M, Burden DA, Lomenick JP, Osheroff N (1997) Quinolones share a common interaction domain on topoisomerase II with other DNA cleavage-enhancing antineoplastic drugs. Biochemistry 36(10):2919–2924
Fan W, Johnson KR, Miller MC 3rd (1998) In vitro evaluation of combination chemotherapy against human tumor cells (Review). Oncol Rep 5(5):1035–1042
Gewirtz DA (1999) A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol 57(7):727–741
Gonzalez MA, Uribe F, Moisen SD, Fuster AP, Selen A, Welling PG, Painter B (1984) Multiple-dose pharmacokinetics and safety of ciprofloxacin in normal volunteers. Antimicrob Agents Chemother 26(5):741–744
Goodin S, Rao KV, DiPaola RS (2002) State-of-the-art treatment of metastatic hormone-refractory prostate cancer. Oncologist 7(4):360–370
Harris KA, Harney E, Small EJ (2002) Liposomal doxorubicin for the treatment of hormone-refractory prostate cancer. Clin Prostate Cancer 1(1):37–41
Hortobagyi GN (1997) Anthracyclines in the treatment of cancer. An overview. Drugs 54(Suppl 4):1–7
Hour TC, Chen J, Huang CY, Guan JY, Lu SH, Pu YS (2002) Curcumin enhances cytotoxicity of chemotherapeutic agents in prostate cancer cells by inducing p21(WAF1/CIP1) and C/EBPbeta expressions and suppressing NF-kappaB activation. Prostate 51(3):211–218
Hubert A, Lyass O, Pode D, Gabizon A (2000) Doxil (Caelyx): an exploratory study with pharmacokinetics in patients with hormone-refractory prostate cancer. Anticancer Drugs 11(2):123–127
Immordino ML, Brusa P, Arpicco S, Stella B, Dosio F, Cattel L (2003) Preparation, characterization, cytotoxicity and pharmacokinetics of liposomes containing docetaxel. J Control Release 91(3):417–429
Kamat AM, DeHaven JI, Lamm DL (1999) Quinolone antibiotics: a potential adjunct to intravesical chemotherapy for bladder cancer. Urology 54(1):56–61
Kaufmann SH, Peereboom D, Buckwalter CA, Svingen PA, Grochow LB, Donehower RC, Rowinsky EK (1996) Cytotoxic effects of topotecan combined with various anticancer agents in human cancer cell lines. J Natl Cancer Inst 88(11):734–741
Kreis W, Budman DR, Calabro A (2001) A reexamination of PSC 833 (Valspodar) as a cytotoxic agent and in combination with anticancer agents. Cancer Chemother Pharmacol 47(1):78–82
Mackler NJ, Pienta KJ (2005) Drug insight: Use of docetaxel in prostate and urothelial cancers. Nat Clin Pract Urol 2(2):92–100 quiz 101 p following 112
Malonne H, Atassi G (1997) DNA topoisomerase targeting drugs: mechanisms of action and perspectives. Anticancer Drugs 8(9):811–822
Naber KG, Sorgel F, Kees F, Jaehde U, Schumacher H (1989) Pharmacokinetics of ciprofloxacin in young (healthy volunteers) and elderly patients, and concentrations in prostatic fluid, seminal fluid, and prostatic adenoma tissue following intravenous administration. Am J Med 87(5A):57S–59S
O’Brien J, Wilson I, Orton T, Pognan F (2000) Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem 267(17):5421–5426
Robinson MJ, Martin BA, Gootz TD, McGuirk PR, Moynihan M, Sutcliffe JA, Osheroff N (1991) Effects of quinolone derivatives on eukaryotic topoisomerase II. A novel mechanism for enhancement of enzyme-mediated DNA cleavage. J Biol Chem 266(22):14585–14592
Simoes S, Slepushkin V, Pires P, Gaspar R, de Lima MP, Duzgunes N (1999) Mechanisms of gene transfer mediated by lipoplexes associated with targeting ligands or pH-sensitive peptides. Gene Ther 6(11):1798–1807
Sonpavde G, Hutson TE, Berry WR (2006) Hormone refractory prostate cancer: management and advances. Cancer Treat Rev 32(2):90–100
van Brussel JP, van Steenbrugge GJ, Romijn JC, Schroder FH, Mickisch GH (1999) Chemosensitivity of prostate cancer cell lines and expression of multidrug resistance-related proteins. Eur J Cancer 35(4):664–671
Wampler GL, Carter WH Jr, Campbell ED, Keefe PA (1992) Relationships between various uses of antineoplastic drug-interaction terms. Cancer Chemother Pharmacol 31(2):111–117
Wientjes MG, Zheng JH, Hu L, Gan Y, Au JL (2005) Intraprostatic chemotherapy: distribution and transport mechanisms. Clin Cancer Res 11(11):4204–4211
Zeng S, Chen YZ, Fu L, Johnson KR, Fan W (2000) In vitro evaluation of schedule-dependent interactions between docetaxel and doxorubicin against human breast and ovarian cancer cells. Clin Cancer Res 6(9):3766–3773
Zoli W, Ricotti L, Tesei A, Barzanti F, Amadori D (2001) In vitro preclinical models for a rational design of chemotherapy combinations in human tumors. Crit Rev Oncol Hematol 37(1):69–82
Acknowledgments
This work was supported by a grant (SFRH/BDE/15519/2004) from Foundation for Science and Technology (FCT) (Portugal) and from Bluepharma, Pharmaceutical Industry SA (Portugal).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pinto, A.C., Moreira, J.N. & Simões, S. Ciprofloxacin sensitizes hormone-refractory prostate cancer cell lines to doxorubicin and docetaxel treatment on a schedule-dependent manner. Cancer Chemother Pharmacol 64, 445–454 (2009). https://doi.org/10.1007/s00280-008-0892-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-008-0892-6