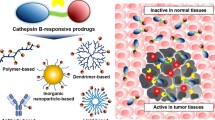
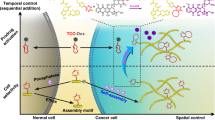
Abstract
The use of conventional chemotherapy in the treatment of cancer has been restricted by the lack of cell specificity, which causes toxicity regarding healthy cells resulting in limiting side effects responsible for low therapeutic efficiency. To overcome these drawbacks, the design of prodrugs has evolved and improved by covalently linking the drug through a degradable spacer. The use of these prodrugs as drug delivery systems, which are able to inactivate the drug during its biodistribution to specifically deliver the drug to its target, is an important breakthrough in cancer therapy. This strategy consisting in the covalent binding of a promoiety to daily used therapeutic compounds has been clinically proven in the design of targeted prodrugs leading enhanced therapeutic efficacy and increase of the therapeutic index. This review summarizes and compares several strategies that improve the therapeutic index of chemotherapy (i.e. conventional drugs) by their chemical transformation into prodrugs improving pharmacokinetic profiles and optimizing administration routes in comparison to the initial drug. This review provides an overview of the methods used to control the structure and function of prodrugs and, ultimately, their current and future potential in increasing the therapeutic index of daily used anticancer drugs. First, prodrugs’ design and their activation within the tumor microenvironment or within the tumor cell will be exposed. Then, the different strategies used leading to these prodrugs will be presented.





Similar content being viewed by others
References
Lesniewska-Kowiel MA, Muszalska I (2017) Strategies in the designing of prodrugs, taking into account the antiviral and anticancer compounds. Eur J Med Chem 129:53–71. https://doi.org/10.1016/j.ejmech.2017.02.011
Singh Y, Palombo M, Sinko PJ (2008) Recent trends in targeted anticancer prodrug and conjugate design. Curr Med Chem 15(18):1802–1826
Soyez H, Schacht E, Vanderkerken S (1996) The crucial role of spacer groups in macromolecular prodrug design. Adv Drug Deliv Rev 21(2):81–106. https://doi.org/10.1016/s0169-409x(96)00400-0
Muller CE (2009) Prodrug approaches for enhancing the bioavailability of drugs with low solubility. Chem Biodivers 6(11):2071–2083. https://doi.org/10.1002/cbdv.200900114
Heynick F (2009) The original ‘magic bullet’ is 100 years old—extra. Br J Psychiatry 195(5):456. https://doi.org/10.1192/bjp.195.5.456
Albert A (1958) Chemical aspects of selective toxicity. Nature 182(4633):421–422. https://doi.org/10.1038/182421a0
Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Jarvinen T, Savolainen J (2008) Prodrugs: design and clinical applications. Nat Rev Drug Discov 7(3):255–270. https://doi.org/10.1038/nrd2468
Mahato R, Tai W, Cheng K (2011) Prodrugs for improving tumor targetability and efficiency. Adv Drug Deliv Rev 63(8):659–670. https://doi.org/10.1016/j.addr.2011.02.002
Gupta D, Gupta SV, Lee KD, Amidon GL (2009) Chemical and enzymatic stability of amino acid prodrugs containing methoxy, ethoxy and propylene glycol linkers. Mol Pharm 6(5):1604–1611. https://doi.org/10.1021/mp900084v
Walther R, Rautio J, Zelikin AN (2017) Prodrugs in medicinal chemistry and enzyme prodrug therapies. Adv Drug Deliv Rev 118:65–77. https://doi.org/10.1016/j.addr.2017.06.013
Alouane A, Labruere R, Le Saux T, Schmidt F, Jullien L (2015) Self-immolative spacers: kinetic aspects, structure–property relationships, and applications. Angew Chem Int Ed Engl 54(26):7492–7509. https://doi.org/10.1002/anie.201500088
Ye M, Han Y, Tang J, Piao Y, Liu X, Zhou Z, Gao J, Rao J, Shen Y (2017) A tumor-specific cascade amplification drug release nanoparticle for overcoming multidrug resistance in cancers. Adv Mater 29(38):1702342. https://doi.org/10.1002/adma.201702342
Bildstein L, Pili B, Marsaud V, Wack S, Meneau F, Lepetre-Mouelhi S, Desmaele D, Bourgaux C, Couvreur P, Dubernet C (2011) Interaction of an amphiphilic squalenoyl prodrug of gemcitabine with cellular membranes. Eur J Pharm Biopharm 79(3):612–620. https://doi.org/10.1016/j.ejpb.2011.07.003
Blanco E, Shen H, Ferrari M (2015) Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol 33(9):941–951. https://doi.org/10.1038/nbt.3330
Maeda H, Nakamura H, Fang J (2013) The EPR effect for macromolecular drug delivery to solid tumors: improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev 65(1):71–79. https://doi.org/10.1016/j.addr.2012.10.002
Greish K (2010) enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. In: Grobmyer SR, Moudgil BM (eds) Cancer nanotechnology: methods and protocols. Humana Press, Totowa, pp 25–37. https://doi.org/10.1007/978-1-60761-609-2_3
Nichols JW, Bae YH (2014) EPR: evidence and fallacy. J Control Release 190:451–464. https://doi.org/10.1016/j.jconrel.2014.03.057
Bansal R, Post E, Proost JH, de Jager-Krikken A, Poelstra K, Prakash J (2011) PEGylation improves pharmacokinetic profile, liver uptake and efficacy of interferon gamma in liver fibrosis. J Control Release 154(3):233–240. https://doi.org/10.1016/j.jconrel.2011.05.027
Choi KY, Min KH, Yoon HY, Kim K, Park JH, Kwon IC, Choi K, Jeong SY (2011) PEGylation of hyaluronic acid nanoparticles improves tumor targetability in vivo. Biomaterials 32(7):1880–1889. https://doi.org/10.1016/j.biomaterials.2010.11.010
Piktel E, Niemirowicz K, Watek M, Wollny T, Deptula P, Bucki R (2016) Recent insights in nanotechnology-based drugs and formulations designed for effective anti-cancer therapy. J Nanobiotechnol 14(1):39. https://doi.org/10.1186/s12951-016-0193-x
Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC (2012) Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev 41(7):2971–3010. https://doi.org/10.1039/c2cs15344k
Khandare JJ, Jayant S, Singh A, Chandna P, Wang Y, Vorsa N, Minko T (2006) Dendrimer versus linear conjugate: influence of polymeric architecture on the delivery and anticancer effect of paclitaxel. Bioconjug Chem 17(6):1464–1472. https://doi.org/10.1021/bc060240p
Langer CJ, O’Byrne KJ, Socinski MA, Mikhailov SM, Lesniewski-Kmak K, Smakal M, Ciuleanu TE, Orlov SV, Dediu M, Heigener D, Eisenfeld AJ, Sandalic L, Oldham FB, Singer JW, Ross HJ (2008) Phase III trial comparing paclitaxel poliglumex (CT-2103, PPX) in combination with carboplatin versus standard paclitaxel and carboplatin in the treatment of PS 2 patients with chemotherapy-naive advanced non-small cell lung cancer. J Thorac Oncol 3(6):623–630. https://doi.org/10.1097/JTO.0b013e3181753b4b
Cheng C, Jiang-Ling Z, Xue H, Fei S, Xiu-Li W, Yu-Zhong W (2014) A prodrug strategy based on chitosan for efficient intracellular anticancer drug delivery. Nanotechnology 25(25):255101
Dragojevic S, Ryu JS, Raucher D (2015) Polymer-based prodrugs: improving tumor targeting and the solubility of small molecule drugs in cancer therapy. Molecules 20(12):21750–21769. https://doi.org/10.3390/molecules201219804
Fumagalli G, Marucci C, Christodoulou MS, Stella B, Dosio F, Passarella D (2016) Self-assembly drug conjugates for anticancer treatment. Drug Discov Today 21(8):1321–1329. https://doi.org/10.1016/j.drudis.2016.06.018
Zhou M, Zhang RH, Wang M, Xu GB, Liao SG (2017) Prodrugs of triterpenoids and their derivatives. Eur J Med Chem 131:222–236. https://doi.org/10.1016/j.ejmech.2017.03.005
Maksimenko A, Dosio F, Mougin J, Ferrero A, Wack S, Reddy LH, Weyn AA, Lepeltier E, Bourgaux C, Stella B, Cattel L, Couvreur P (2014) A unique squalenoylated and nonpegylated doxorubicin nanomedicine with systemic long-circulating properties and anticancer activity. Proc Natl Acad Sci USA 111(2):E217–E226. https://doi.org/10.1073/pnas.1313459110
Mura S, Nicolas J, Couvreur P (2013) Stimuli-responsive nanocarriers for drug delivery. Nat Mater 12(11):991–1003. https://doi.org/10.1038/nmat3776
Siafaka PI, Ustundag Okur N, Karavas E, Bikiaris DN (2016) Surface modified multifunctional and stimuli responsive nanoparticles for drug targeting: current status and uses. Int J Mol Sci. https://doi.org/10.3390/ijms17091440
Chau Y, Padera RF, Dang NM, Langer R (2006) Antitumor efficacy of a novel polymer-peptide-drug conjugate in human tumor xenograft models. Int J Cancer 118(6):1519–1526. https://doi.org/10.1002/ijc.21495
Huang C, Yi X, Kong D, Chen L, Min G (2016) Controlled release strategy of paclitaxel by conjugating to matrix metalloproteinases-2 sensitive peptide. Oncotarget 7(32):52230–52238. https://doi.org/10.18632/oncotarget.10735
Denmeade SR, Nagy A, Gao J, Lilja H, Schally AV, Isaacs JT (1998) Enzymatic activation of a doxorubicin-peptide prodrug by prostate-specific antigen. Cancer Res 58(12):2537–2540
DiPaola RS, Rinehart J, Nemunaitis J, Ebbinghaus S, Rubin E, Capanna T, Ciardella M, Doyle-Lindrud S, Goodwin S, Fontaine M, Adams N, Williams A, Schwartz M, Winchell G, Wickersham K, Deutsch P, Yao SL (2002) Characterization of a novel prostate-specific antigen-activated peptide-doxorubicin conjugate in patients with prostate cancer. J Clin Oncol 20(7):1874–1879. https://doi.org/10.1200/JCO.2002.07.001
Wu X, Hu L (2016) Design and synthesis of peptide conjugates of phosphoramide mustard as prodrugs activated by prostate-specific antigen. Bioorg Med Chem 24(12):2697–2706. https://doi.org/10.1016/j.bmc.2016.04.035
DeFeo-Jones D, Brady SF, Feng DM, Wong BK, Bolyar T, Haskell K, Kiefer DM, Leander K, McAvoy E, Lumma P, Pawluczyk JM, Wai J, Motzel SL, Keenan K, Van Zwieten M, Lin JH, Garsky VM, Freidinger R, Oliff A, Jones RE (2002) A prostate-specific antigen (PSA)-activated vinblastine prodrug selectively kills PSA-secreting cells in vivo. Mol Cancer Ther 1(7):451–459
Brennen WN, Rosen DM, Wang H, Isaacs JT, Denmeade SR (2012) Targeting carcinoma-associated fibroblasts within the tumor stroma with a fibroblast activation protein-activated prodrug. J Natl Cancer Inst 104(17):1320–1334. https://doi.org/10.1093/jnci/djs336
Chen M, Lei X, Shi C, Huang M, Li X, Wu B, Li Z, Han W, Du B, Hu J, Nie Q, Mai W, Ma N, Xu N, Zhang X, Fan C, Hong A, Xia M, Luo L, Ma A, Li H, Yu Q, Chen H, Zhang D, Ye W (2017) Pericyte-targeting prodrug overcomes tumor resistance to vascular disrupting agents. J Clin Investig 127(10):3689–3701. https://doi.org/10.1172/JCI94258
Huang S, Zhang Y, Zhong J, Pan Y, Cai S, Xu J (2018) Toxicological profile and safety pharmacology of a single dose of fibroblast activation protein-alpha-based doxorubicin prodrug: in vitro and in vivo evaluation. Anticancer Drugs 29(3):253–261. https://doi.org/10.1097/CAD.0000000000000593
Wang J, Li Q, Li X, Yuan W, Huang S, Cai S, Xu J (2017) A novel FAPalpha-based Z-Gly-Pro epirubicin prodrug for improving tumor-targeting chemotherapy. Eur J Pharmacol 815:166–172. https://doi.org/10.1016/j.ejphar.2017.09.016
Aggarwal N, Sloane BF (2014) Cathepsin B: multiple roles in cancer. Proteom Clin Appl 8(5–6):427–437. https://doi.org/10.1002/prca.201300105
Shao LH, Liu SP, Hou JX, Zhang YH, Peng CW, Zhong YJ, Liu X, Liu XL, Hong YP, Firestone RA, Li Y (2012) Cathepsin B cleavable novel prodrug Ac-Phe-Lys-PABC-ADM enhances efficacy at reduced toxicity in treating gastric cancer peritoneal carcinomatosis: an experimental study. Cancer 118(11):2986–2996. https://doi.org/10.1002/cncr.26596
Zhong YJ, Shao LH, Li Y (2013) Cathepsin B-cleavable doxorubicin prodrugs for targeted cancer therapy (review). Int J Oncol 42(2):373–383. https://doi.org/10.3892/ijo.2012.1754
Seymour LW, Ferry DR, Kerr DJ, Rea D, Whitlock M, Poyner R, Boivin C, Hesslewood S, Twelves C, Blackie R, Schatzlein A, Jodrell D, Bissett D, Calvert H, Lind M, Robbins A, Burtles S, Duncan R, Cassidy J (2009) Phase II studies of polymer-doxorubicin (PK1, FCE28068) in the treatment of breast, lung and colorectal cancer. Int J Oncol 34(6):1629–1636. https://doi.org/10.3892/ijo_00000293
Paz-Ares L, Ross H, O’Brien M, Riviere A, Gatzemeier U, Von Pawel J, Kaukel E, Freitag L, Digel W, Bischoff H, Garcia-Campelo R, Iannotti N, Reiterer P, Bover I, Prendiville J, Eisenfeld AJ, Oldham FB, Bandstra B, Singer JW, Bonomi P (2008) Phase III trial comparing paclitaxel poliglumex vs docetaxel in the second-line treatment of non-small-cell lung cancer. Br J Cancer 98(10):1608–1613. https://doi.org/10.1038/sj.bjc.6604372
Zhang M, Jiang Z, Chen S, Wu Z, Chen K, Wu Y (2018) Legumain correlates with neuroblastoma differentiation and can be used in prodrug design. Chem Biol Drug Des 91(2):534–544. https://doi.org/10.1111/cbdd.13116
Connors JMRJ (2018) Brentuximab vedotin for stage III or IV Hodgkin’s lymphoma. N Engl J Med 378(16):1558–1561. https://doi.org/10.1056/NEJMc1802363
Karnthaler-Benbakka C, Koblmuller B, Mathuber M, Holste K, Berger W, Heffeter P, Kowol CR, Keppler BK (2018) Synthesis, characterization and in vitro studies of a Cathepsin B-cleavable prodrug of the VEGFR inhibitor sunitinib. Chem Biodivers. https://doi.org/10.1002/cbdv.201800520
Bollag W (1965) Hartmann HR (1980) tumor inhibitory effects of a new fluorouracil derivative: 5′-deoxy-5-fluorouridine. Eur J Cancer 16(4):427–432. https://doi.org/10.1016/0014-2964(80)90221-2
Hiller SA, Lidak MY, Zhuk RA et al (1969) Analogs of pyrimidine nucleosides. Chem Heterocycl Compd 5(2):283–285. https://doi.org/10.1007/BF00943946
Koukourakis GV, Kouloulias V, Koukourakis MJ, Zacharias GA, Zabatis H, Kouvaris J (2008) Efficacy of the oral fluorouracil pro-drug capecitabine in cancer treatment: a review. Molecules 13(8):1897–1922. https://doi.org/10.3390/molecules13081897
Sakata Y, Ohtsu A, Horikoshi N, Sugimachi K, Mitachi Y, Taguchi T (1998) Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur–0.4 M gimestat–1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer 34(11):1715–1720. https://doi.org/10.1016/s0959-8049(98)00211-1
Alberto P, Winkelmann JJ, Paschoud N, Peytremann R, Bruyere A, Righetti A, Decoster G, Holdener EE (1989) Phase I study of oral doxifluridine using two schedules. Eur J Cancer Clin Oncol 25(5):905–908. https://doi.org/10.1016/0277-5379(89)90139-9
Kim NK, Min JS, Park JK, Yun SH, Sung JS, Jung HC, Roh JK (2001) Intravenous 5-fluorouracil versus oral doxifluridine as preoperative concurrent chemoradiation for locally advanced rectal cancer: prospective randomized trials. Jpn J Clin Oncol 31(1):25–29. https://doi.org/10.1093/jjco/hye009
van Oosterom AT, ten Bokkel Huinink WW, van der Burg ME, Vermorken JB, Willemse PH, Neijt JP (1991) Phase II clinical trial of doxifluridine in patients with advanced ovarian cancer. Eur J Cancer 27(6):747–749. https://doi.org/10.1016/0277-5379(91)90180-L
Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, Shimma N, Umeda I, Ishitsuka H (1998) Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer 34(8):1274–1281. https://doi.org/10.1016/s0959-8049(98)00058-6
Ter Veer E, Ngai LL, Valkenhoef GV, Mohammad NH, Anderegg MCJ, van Oijen MGH, van Laarhoven HWM (2017) Capecitabine, 5-fluorouracil and S-1 based regimens for previously untreated advanced oesophagogastric cancer: a network meta-analysis. Sci Rep 7(1):7142. https://doi.org/10.1038/s41598-017-07750-3
Fukushima M, Iizuka K, Jin C, Zhang C, Hong M, Eshima K (2017) Development of new promising antimetabolite, DFP-11207 with self-controlled toxicity in rodents. Drug Des Dev Ther 11:1693–1705. https://doi.org/10.2147/DDDT.S128420
Zhao D, Zhang H, Tao W, Wei W, Sun J, He Z (2017) A rapid albumin-binding 5-fluorouracil prodrug with a prolonged circulation time and enhanced antitumor activity. Biomater Sci 5(3):502–510. https://doi.org/10.1039/c6bm00884d
Dubois V, Dasnois L, Lebtahi K, Collot F, Heylen N, Havaux N, Fernandez AM, Lobl TJ, Oliyai C, Nieder M, Shochat D, Yarranton GT, Trouet A (2002) CPI-0004Na, a new extracellularly tumor-activated prodrug of doxorubicin: in vivo toxicity, activity, and tissue distribution confirm tumor cell selectivity. Cancer Res 62(8):2327–2331
Schoffski P, Delord JP, Brain E, Robert J, Dumez H, Gasmi J, Trouet A (2017) First-in-man phase I study assessing the safety and pharmacokinetics of a 1-h intravenous infusion of the doxorubicin prodrug DTS-201 every 3 weeks in patients with advanced or metastatic solid tumours. Eur J Cancer 86:240–247. https://doi.org/10.1016/j.ejca.2017.09.009
Cornillie J, Wozniak A, Pokreisz P, Casazza A, Vreys L, Wellens J, Vanleeuw U, Gebreyohannes YK, Debiec-Rychter M, Sciot R, Hompes D, Schoffski P (2017) In vivo antitumoral efficacy of PhAc-ALGP-doxorubicin, an enzyme-activated doxorubicin prodrug, in patient-derived soft tissue sarcoma xenograft models. Mol Cancer Ther 16(8):1566–1575. https://doi.org/10.1158/1535-7163.MCT-16-0832
Vandooren J, Opdenakker G, Loadman PM, Edwards DR (2016) Proteases in cancer drug delivery. Adv Drug Deliv Rev 97:144–155. https://doi.org/10.1016/j.addr.2015.12.020
Wu W, Luo Y, Sun C, Liu Y, Kuo P, Varga J, Xiang R, Reisfeld R, Janda KD, Edgington TS, Liu C (2006) Targeting cell-impermeable prodrug activation to tumor microenvironment eradicates multiple drug-resistant neoplasms. Cancer Res 66(2):970–980. https://doi.org/10.1158/0008-5472.CAN-05-2591
Zhou H, Sun H, Lv S, Zhang D, Zhang X, Tang Z, Chen X (2017) Legumain-cleavable 4-arm poly(ethylene glycol)-doxorubicin conjugate for tumor specific delivery and release. Acta Biomater 54:227–238. https://doi.org/10.1016/j.actbio.2017.03.019
Stern L, Perry R, Ofek P, Many A, Shabat D, Satchi-Fainaro R (2009) A novel antitumor prodrug platform designed to be cleaved by the endoprotease legumain. Bioconjug Chem 20(3):500–510. https://doi.org/10.1021/bc800448u
Zhang H, Sun Z, Wang K, Li N, Chen H, Tan X, Li L, He Z, Sun J (2018) Multifunctional tumor-targeting cathepsin B-sensitive gemcitabine prodrug covalently targets albumin in situ and improves cancer therapy. Bioconjug Chem 29(6):1852–1858. https://doi.org/10.1021/acs.bioconjchem.8b00223
Wu W, Luo Y, Sun C, Liu Y, Kuo P, Varga J, Xiang R, Reisfeld R, Janda KD, Edgington TS, Liu C (2006) Targeting cell-impermeable prodrug activation to tumor microenvironment eradicates multiple drug-resistant neoplasms. Can Res 66(2):970. https://doi.org/10.1158/0008-5472.CAN-05-2591
Seo K, Chung SW, Byun Y, Kim D (2012) Paclitaxel loaded nano-aggregates based on pH sensitive polyaspartamide amphiphilic graft copolymers. Int J Pharm 424(1–2):26–32. https://doi.org/10.1016/j.ijpharm.2011.12.047
Yoshida T, Lai TC, Kwon GS, Sako K (2013) pH- and ion-sensitive polymers for drug delivery. Expert Opin Drug Deliv 10(11):1497–1513. https://doi.org/10.1517/17425247.2013.821978
Xiong S, Wang Z, Liu J, Deng X, Xiong R, Cao X, Xie Z, Lei X, Chen Y, Tang G (2019) A pH-sensitive prodrug strategy to co-deliver DOX and TOS in TPGS nanomicelles for tumor therapy. Colloids Surf B Biointerfaces 173:346–355. https://doi.org/10.1016/j.colsurfb.2018.10.012
Huang X, Liao W, Xie Z, Chen D, Zhang CY (2018) A pH-responsive prodrug delivery system self-assembled from acid-labile doxorubicin-conjugated amphiphilic pH-sensitive block copolymers. Mater Sci Eng C Mater Biol Appl 90:27–37. https://doi.org/10.1016/j.msec.2018.04.036
Rahoui N, Jiang B, Taloub N, Hegazy M, Huang YD (2018) Synthesis and evaluation of water soluble pH sensitive poly (vinyl alcohol)-doxorubicin conjugates. J Biomater Sci Polym Ed 29(12):1482–1497. https://doi.org/10.1080/09205063.2018.1466470
Zhu J, Huo Q, Xu M, Yang F, Li Y, Shi H, Niu Y, Liu Y (2018) Bortezomib-catechol conjugated prodrug micelles: combining bone targeting and aryl boronate-based pH-responsive drug release for cancer bone-metastasis therapy. Nanoscale 10(38):18387–18397. https://doi.org/10.1039/c8nr03899f
Xie J, Fan Z, Li Y, Zhang Y, Yu F, Su G, Xie L, Hou Z (2018) Design of pH-sensitive methotrexate prodrug-targeted curcumin nanoparticles for efficient dual-drug delivery and combination cancer therapy. Int J Nanomed 13:1381–1398. https://doi.org/10.2147/IJN.S152312
Tap WD, Papai Z, Van Tine BA, Attia S, Ganjoo KN, Jones RL, Schuetze S, Reed D, Chawla SP, Riedel RF, Krarup-Hansen A, Toulmonde M, Ray-Coquard I, Hohenberger P, Grignani G, Cranmer LD, Okuno S, Agulnik M, Read W, Ryan CW, Alcindor T, del Muro XFG, Budd GT, Tawbi H, Pearce T, Kroll S, Reinke DK, Schöffski P (2017) Doxorubicin plus evofosfamide versus doxorubicin alone in locally advanced, unresectable or metastatic soft-tissue sarcoma (TH CR-406/SARC021): an international, multicentre, open-label, randomised phase 3 trial. Lancet Oncol 18(8):1089–1103. https://doi.org/10.1016/s1470-2045(17)30381-9
Laubach JP, Liu CJ, Raje NS, Yee AJ, Armand P, Schlossman RL, Rosenblatt J, Hedlund J, Martin M, Reynolds C, Shain KH, Zackon I, Stampleman L, Henrick P, Rivotto B, Hornburg KTV, Dumke HJ, Chuma S, Savell A, Handisides DR, Kroll S, Anderson KC, Richardson PG, Ghobrial IM (2018) A phase I/II study of evofosfamide, a hypoxia-activated prodrug with or without bortezomib in subjects with relapsed/refractory multiple myeloma. Clin Cancer Res. https://doi.org/10.1158/1078-0432.ccr-18-1325
Jamieson SM, Tsai P, Kondratyev MK, Budhani P, Liu A, Senzer NN, Chiorean EG, Jalal SI, Nemunaitis JJ, Kee D, Shome A, Wong WW, Li D, Poonawala-Lohani N, Kakadia PM, Knowlton NS, Lynch CR, Hong CR, Lee TW, Grenman RA, Caporiccio L, McKee TD, Zaidi M, Butt S, Macann AM, McIvor NP, Chaplin JM, Hicks KO, Bohlander SK, Wouters BG, Hart CP, Print CG, Wilson WR, Curran MA, Hunter FW (2018) Evofosfamide for the treatment of human papillomavirus-negative head and neck squamous cell carcinoma. JCI Insight. https://doi.org/10.1172/jci.insight.122204
Kumar S, Sun JD, Zhang L, Mokhtari RB, Wu B, Meng F, Liu Q, Bhupathi D, Wang Y, Yeger H, Hart C, Baruchel S (2018) Hypoxia-targeting drug evofosfamide (TH-302) enhances sunitinib activity in neuroblastoma xenograft models. Transl Oncol 11(4):911–919. https://doi.org/10.1016/j.tranon.2018.05.004
Liu S, Tetzlaff M, Wang T, Chen X, Yang R, Kumar SM, Xu X (2017) Hypoxia-activated prodrug enhances therapeutic effect of sunitinib in melanoma. Oncotarget 8(70):115140–115152. https://doi.org/10.18632/oncotarget.22944
Huang Y, Tian Y, Zhao Y, Xue C, Zhan J, Liu L, He X, Zhang L (2018) Efficacy of the hypoxia-activated prodrug evofosfamide (TH-302) in nasopharyngeal carcinoma in vitro and in vivo. Cancer Commun (Lond) 38(1):15. https://doi.org/10.1186/s40880-018-0285-0
Brenner A, Zuniga R, Sun JD, Floyd J, Hart CP, Kroll S, Fichtel L, Cavazos D, Caflisch L, Gruslova A, Huang S, Liu Y, Lodi A, Tiziani S (2018) Hypoxia-activated evofosfamide for treatment of recurrent bevacizumab-refractory glioblastoma: a phase I surgical study. Neurooncology 20(9):1231–1239. https://doi.org/10.1093/neuonc/noy015
Liapis V, Zysk A, DeNichilo M, Zinonos I, Hay S, Panagopoulos V, Shoubridge A, Difelice C, Ponomarev V, Ingman W, Atkins GJ, Findlay DM, Zannettino ACW, Evdokiou A (2017) Anticancer efficacy of the hypoxia-activated prodrug evofosfamide is enhanced in combination with proapoptotic receptor agonists against osteosarcoma. Cancer Med 6(9):2164–2176. https://doi.org/10.1002/cam4.1115
Hajj C, Russell J, Hart CP, Goodman KA, Lowery MA, Haimovitz-Friedman A, Deasy JO, Humm JL (2017) A combination of radiation and the hypoxia-activated prodrug evofosfamide (TH-302) is efficacious against a human orthotopic pancreatic tumor model. Transl Oncol 10(5):760–765. https://doi.org/10.1016/j.tranon.2017.06.010
Jin C, Zhang Q, Lu W (2017) Synthesis and biological evaluation of hypoxia-activated prodrugs of SN-38. Eur J Med Chem 132:135–141. https://doi.org/10.1016/j.ejmech.2017.03.040
McKeage MJ, Gu Y, Wilson WR, Hill A, Amies K, Melink TJ, Jameson MB (2011) A phase I trial of PR-104, a pre-prodrug of the bioreductive prodrug PR-104A, given weekly to solid tumour patients. BMC Cancer 11:432. https://doi.org/10.1186/1471-2407-11-432
Guise CP, Abbattista MR, Singleton RS, Holford SD, Connolly J, Dachs GU, Fox SB, Pollock R, Harvey J, Guilford P, Donate F, Wilson WR, Patterson AV (2010) The bioreductive prodrug PR-104A is activated under aerobic conditions by human aldo-keto reductase 1C3. Cancer Res 70(4):1573–1584. https://doi.org/10.1158/0008-5472.CAN-09-3237
Konopleva M, Thall PF, Yi CA, Borthakur G, Coveler A, Bueso-Ramos C, Benito J, Konoplev S, Gu Y, Ravandi F, Jabbour E, Faderl S, Thomas D, Cortes J, Kadia T, Kornblau S, Daver N, Pemmaraju N, Nguyen HQ, Feliu J, Lu H, Wei C, Wilson WR, Melink TJ, Gutheil JC, Andreeff M, Estey EH, Kantarjian H (2015) Phase I/II study of the hypoxia-activated prodrug PR104 in refractory/relapsed acute myeloid leukemia and acute lymphoblastic leukemia. Haematologica 100(7):927–934. https://doi.org/10.3324/haematol.2014.118455
Peng X, Gandhi V (2012) ROS-activated anticancer prodrugs: a new strategy for tumor-specific damage. Ther Deliv 3(7):823–833. https://doi.org/10.4155/tde.12.61
Saravanakumar G, Kim J, Kim Won J (2016) Reactive-oxygen-species-responsive drug delivery systems: promises and challenges. Adv Sci 4(1):1600124. https://doi.org/10.1002/advs.201600124
Kuang Y, Balakrishnan K, Gandhi V, Peng X (2011) Hydrogen peroxide inducible DNA cross-linking agents: targeted anticancer prodrugs. J Am Chem Soc 133(48):19278–19281. https://doi.org/10.1021/ja2073824
Wang L, Xie S, Ma L, Chen Y, Lu W (2016) 10-Boronic acid substituted camptothecin as prodrug of SN-38. Eur J Med Chem 116:84–89. https://doi.org/10.1016/j.ejmech.2016.03.063
Zhang C, Zhong Q, Zhang Q, Zheng S, Miele L, Wang G (2015) Boronic prodrug of endoxifen as an effective hormone therapy for breast cancer. Breast Cancer Res Treat 152(2):283–291. https://doi.org/10.1007/s10549-015-3461-9
Gabor F, Wollmann K, Theyer G, Haberl I, Hamilton G (1994) In vitro antiproliferative effects of albumin-doxorubicin conjugates against Ewing’s sarcoma and peripheral neuroectodermal tumor cells. Anticancer Res 14(5A):1943–1950
Kratz F (2007) DOXO-EMCH (INNO-206): the first albumin-binding prodrug of doxorubicin to enter clinical trials. Expert Opin Investig Drugs 16(6):855–866. https://doi.org/10.1517/13543784.16.6.855
Chawla SP, Papai Z, Mukhametshina G, Sankhala K, Vasylyev L, Fedenko A, Khamly K, Ganjoo K, Nagarkar R, Wieland S, Levitt DJ (2015) First-line aldoxorubicin vs doxorubicin in metastatic or locally advanced unresectable soft-tissue sarcoma: a phase 2b randomized clinical trial. JAMA Oncol 1(9):1272–1280. https://doi.org/10.1001/jamaoncol.2015.3101
Mita MM, Natale RB, Wolin EM, Laabs B, Dinh H, Wieland S, Levitt DJ, Mita AC (2015) Pharmacokinetic study of aldoxorubicin in patients with solid tumors. Investig New Drugs 33(2):341–348. https://doi.org/10.1007/s10637-014-0183-5
Mansour AM, Drevs J, Esser N, Hamada FM, Badary OA, Unger C, Fichtner I, Kratz F (2003) A new approach for the treatment of malignant melanoma: enhanced antitumor efficacy of an albumin-binding doxorubicin prodrug that is cleaved by matrix metalloproteinase 2. Can Res 63(14):4062
Schmid B, Chung DE, Warnecke A, Fichtner I, Kratz F (2007) Albumin-binding prodrugs of camptothecin and doxorubicin with an Ala-Leu-Ala-Leu-linker that are cleaved by cathepsin B: synthesis and antitumor efficacy. Bioconjug Chem 18(3):702–716. https://doi.org/10.1021/bc0602735
Graeser R, Chung DE, Esser N, Moor S, Schachtele C, Unger C, Kratz F (2008) Synthesis and biological evaluation of an albumin-binding prodrug of doxorubicin that is cleaved by prostate-specific antigen (PSA) in a PSA-positive orthotopic prostate carcinoma model (LNCaP). Int J Cancer 122(5):1145–1154. https://doi.org/10.1002/ijc.23050
Wunder A, Stehle G, Schrenk HH, Hartung G, Heene DL, Maier-Borst W, Sinn H (1998) Antitumor activity of methotrexate-albumin conjugates in rats bearing a Walker-256 carcinoma. Int J Cancer 76(6):884–890. https://doi.org/10.1002/(SICI)1097-0215(19980610)76:6%3c884:AID-IJC19%3e3.0.CO;2-2
Hartung G, Stehle G, Sinn H, Wunder A, Schrenk HH, Heeger S, Kranzle M, Edler L, Frei E, Fiebig HH, Heene DL, Maier-Borst W, Queisser W (1999) Phase I trial of methotrexate-albumin in a weekly intravenous bolus regimen in cancer patients. Phase I Study Group of the Association for Medical Oncology of the German Cancer Society. Clin Cancer Res 5(4):753–759
Vis AN, van der Gaast A, van Rhijn BW, Catsburg TK, Schmidt C, Mickisch GH (2002) A phase II trial of methotrexate-human serum albumin (MTX-HSA) in patients with metastatic renal cell carcinoma who progressed under immunotherapy. Cancer Chemother Pharmacol 49(4):342–345. https://doi.org/10.1007/s00280-001-0417-z
Mayr J, Heffeter P, Groza D, Galvez L, Koellensperger G, Roller A, Alte B, Haider M, Berger W, Kowol CR, Keppler BK (2017) An albumin-based tumor-targeted oxaliplatin prodrug with distinctly improved anticancer activity in vivo. Chem Sci 8(3):2241–2250. https://doi.org/10.1039/c6sc03862j
Beck A, D’Atri V, Ehkirch A, Fekete S, Hernandez-Alba O, Gahoual R, Leize-Wagner E, Francois Y, Guillarme D, Cianferani S (2019) Cutting-edge multi-level analytical and structural characterization of antibody–drug conjugates: present and future. Expert Rev Proteom 16(4):337–362. https://doi.org/10.1080/14789450.2019.1578215
Jen EY, Ko CW, Lee JE, Del Valle PL, Aydanian A, Jewell C, Norsworthy KJ, Przepiorka D, Nie L, Liu J, Sheth CM, Shapiro M, Farrell AT, Pazdur R (2018) FDA approval: gemtuzumab ozogamicin for the treatment of adults with newly diagnosed CD33-positive acute myeloid leukemia. Clin Cancer Res 24(14):3242–3246. https://doi.org/10.1158/1078-0432.CCR-17-3179
Tack DK, Letendre L, Kamath PS, Tefferi A (2001) Development of hepatic veno-occlusive disease after Mylotarg infusion for relapsed acute myeloid leukemia. Bone Marrow Transplant 28(9):895–897. https://doi.org/10.1038/sj.bmt.1703242
Lu J, Jiang F, Lu A, Zhang G (2016) Linkers having a crucial role in antibody–drug conjugates. Int J Mol Sci 17(4):561. https://doi.org/10.3390/ijms17040561
Junttila TT, Li G, Parsons K, Phillips GL, Sliwkowski MX (2011) Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat 128(2):347–356. https://doi.org/10.1007/s10549-010-1090-x
Girish S, Gupta M, Wang B, Lu D, Krop IE, Vogel CL, Burris Iii HA, LoRusso PM, Yi J-H, Saad O, Tong B, Chu Y-W, Holden S, Joshi A (2012) Clinical pharmacology of trastuzumab emtansine (T-DM1): an antibody–drug conjugate in development for the treatment of HER2-positive cancer. Cancer Chemother Pharmacol 69(5):1229–1240. https://doi.org/10.1007/s00280-011-1817-3
Joubert N, Denevault-Sabourin C, Bryden F, Viaud-Massuard MC (2017) Towards antibody–drug conjugates and prodrug strategies with extracellular stimuli-responsive drug delivery in the tumor microenvironment for cancer therapy. Eur J Med Chem 142:393–415. https://doi.org/10.1016/j.ejmech.2017.08.049
Chen H, Lin Z, Arnst KE, Miller DD, Li W (2017) Tubulin inhibitor-based antibody–drug conjugates for cancer therapy. Molecules. https://doi.org/10.3390/molecules22081281
Riechelmann H, Sauter A, Golze W, Hanft G, Schroen C, Hoermann K, Erhardt T, Gronau S (2008) Phase I trial with the CD44v6-targeting immunoconjugate bivatuzumab mertansine in head and neck squamous cell carcinoma. Oral Oncol 44(9):823–829. https://doi.org/10.1016/j.oraloncology.2007.10.009
Dal Corso A, Cazzamalli S, Gebleux R, Mattarella M, Neri D (2017) Protease-cleavable linkers modulate the anticancer activity of noninternalizing antibody–drug conjugates. Bioconjug Chem 28(7):1826–1833. https://doi.org/10.1021/acs.bioconjchem.7b00304
Perrino E, Steiner M, Krall N, Bernardes GJ, Pretto F, Casi G, Neri D (2014) Curative properties of noninternalizing antibody–drug conjugates based on maytansinoids. Cancer Res 74(9):2569–2578. https://doi.org/10.1158/0008-5472.CAN-13-2990
Richards DA (2018) Exploring alternative antibody scaffolds: antibody fragments and antibody mimics for targeted drug delivery. Drug Discov Today Technol 30:35–46. https://doi.org/10.1016/j.ddtec.2018.10.005
Aubrey N, Allard-Vannier E, Martin C, Bryden F, Letast S, Colas C, Lakhrif Z, Collinet N, Dimier-Poisson I, Chourpa I, Viaud-Massuard MC, Joubert N (2018) Site-specific conjugation of auristatins onto engineered scFv using second generation maleimide to target HER2-positive breast cancer in vitro. Bioconjug Chem 29(11):3516–3521. https://doi.org/10.1021/acs.bioconjchem.8b00668
Desgrosellier JS, Cheresh DA (2010) Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 10(1):9–22. https://doi.org/10.1038/nrc2748
Dechantsreiter MA, Planker E, Matha B, Lohof E, Holzemann G, Jonczyk A, Goodman SL, Kessler H (1999) N-Methylated cyclic RGD peptides as highly active and selective alpha(V)beta(3) integrin antagonists. J Med Chem 42(16):3033–3040. https://doi.org/10.1021/jm970832g
Massaguer A, Gonzalez-Canto A, Escribano E, Barrabes S, Artigas G, Moreno V, Marchan V (2015) Integrin-targeted delivery into cancer cells of a Pt(IV) pro-drug through conjugation to RGD-containing peptides. Dalton Trans 44(1):202–212. https://doi.org/10.1039/c4dt02710h
Dal Pozzo A, Esposito E, Ni M, Muzi L, Pisano C, Bucci F, Vesci L, Castorina M, Penco S (2010) Conjugates of a novel 7-substituted camptothecin with RGD-peptides as alpha(v)beta(3) integrin ligands: an approach to tumor-targeted therapy. Bioconjug Chem 21(11):1956–1967. https://doi.org/10.1021/bc100097r
Arosio D, Casagrande C (2016) Advancement in integrin facilitated drug delivery. Adv Drug Deliv Rev 97:111–143. https://doi.org/10.1016/j.addr.2015.12.001
Zhong P, Gu X, Cheng R, Deng C, Meng F, Zhong Z (2017) alphavbeta3 integrin-targeted micellar mertansine prodrug effectively inhibits triple-negative breast cancer in vivo. Int J Nanomed 12:7913–7921. https://doi.org/10.2147/IJN.S146505
Engel J, Emons G, Pinski J, Schally AV (2012) AEZS-108: a targeted cytotoxic analog of LHRH for the treatment of cancers positive for LHRH receptors. Expert Opin Investig Drugs 21(6):891–899. https://doi.org/10.1517/13543784.2012.685128
Emons G, Gorchev G, Sehouli J, Wimberger P, Stahle A, Hanker L, Hilpert F, Sindermann H, Grundker C, Harter P (2014) Efficacy and safety of AEZS-108 (INN: zoptarelin doxorubicin acetate) an LHRH agonist linked to doxorubicin in women with platinum refractory or resistant ovarian cancer expressing LHRH receptors: a multicenter phase II trial of the ago-study group (AGO GYN 5). Gynecol Oncol 133(3):427–432. https://doi.org/10.1016/j.ygyno.2014.03.576
Liu SV, Tsao-Wei DD, Xiong S, Groshen S, Dorff TB, Quinn DI, Tai YC, Engel J, Hawes D, Schally AV, Pinski JK (2014) Phase I, dose-escalation study of the targeted cytotoxic LHRH analog AEZS-108 in patients with castration- and taxane-resistant prostate cancer. Clin Cancer Res 20(24):6277–6283. https://doi.org/10.1158/1078-0432.CCR-14-0489
Yu SS, Athreya K, Liu SV, Schally AV, Tsao-Wei D, Groshen S, Quinn DI, Dorff TB, Xiong S, Engel J, Pinski J (2017) A phase II trial of AEZS-108 in castration- and taxane-resistant prostate cancer. Clin Genitourin Cancer 15(6):742–749. https://doi.org/10.1016/j.clgc.2017.06.002
Karampelas T, Skavatsou E, Argyros O, Fokas D, Tamvakopoulos C (2017) Gemcitabine based peptide conjugate with improved metabolic properties and dual mode of efficacy. Mol Pharm 14(3):674–685. https://doi.org/10.1021/acs.molpharmaceut.6b00961
Kurzrock R, Gabrail N, Chandhasin C, Moulder S, Smith C, Brenner A, Sankhala K, Mita A, Elian K, Bouchard D, Sarantopoulos J (2012) Safety, pharmacokinetics, and activity of GRN1005, a novel conjugate of angiopep-2, a peptide facilitating brain penetration, and paclitaxel, in patients with advanced solid tumors. Mol Cancer Ther 11(2):308–316. https://doi.org/10.1158/1535-7163.MCT-11-0566
Drappatz J, Brenner A, Wong ET, Eichler A, Schiff D, Groves MD, Mikkelsen T, Rosenfeld S, Sarantopoulos J, Meyers CA, Fielding RM, Elian K, Wang X, Lawrence B, Shing M, Kelsey S, Castaigne JP, Wen PY (2013) Phase I study of GRN1005 in recurrent malignant glioma. Clin Cancer Res 19(6):1567–1576. https://doi.org/10.1158/1078-0432.CCR-12-2481
Gelderblom H, Verweij J, Nooter K, Sparreboom A (2001) Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer 37(13):1590–1598. https://doi.org/10.1016/S0959-8049(01)00171-X
Huo M, Zhu Q, Wu Q, Yin T, Wang L, Yin L, Zhou J (2015) Somatostatin receptor-mediated specific delivery of paclitaxel prodrugs for efficient cancer therapy. J Pharm Sci 104(6):2018–2028. https://doi.org/10.1002/jps.24438
Steinberg G, Borch RF (2001) Synthesis and evaluation of pteroic acid-conjugated nitroheterocyclic phosphoramidates as folate receptor-targeted alkylating agents. J Med Chem 44(1):69–73. https://doi.org/10.1021/jm000306g
Aronov O, Horowitz AT, Gabizon A, Gibson D (2003) Folate-targeted PEG as a potential carrier for carboplatin analogs. Synthesis and in vitro studies. Bioconjug Chem 14(3):563–574. https://doi.org/10.1021/bc025642l
Liu J, Kolar C, Lawson TA, Gmeiner WH (2002) Targeted drug delivery to chemoresistant cells: folic acid derivatization of FdUMP[10] enhances cytotoxicity toward 5-FU-Resistant human colorectal tumor cells. J Org Chem 67(8):2734. https://doi.org/10.1021/jo0161983
Lee JW, Lu JY, Low PS, Fuchs PL (2002) Synthesis and evaluation of taxol–folic acid conjugates as targeted antineoplastics. Bioorg Med Chem 10(7):2397–2414. https://doi.org/10.1016/s0968-0896(02)00019-6
Ladino CA, Chari RV, Bourret LA, Kedersha NL, Goldmacher VS (1997) Folate-maytansinoids: target-selective drugs of low molecular weight. Int J Cancer 73(6):859–864. https://doi.org/10.1002/(SICI)1097-0215(19971210)73:6%3c859:AID-IJC16%3e3.0.CO;2-%23
Reddy JA, Westrick E, Santhapuram HK, Howard SJ, Miller ML, Vetzel M, Vlahov I, Chari RV, Goldmacher VS, Leamon CP (2007) Folate receptor-specific antitumor activity of EC131, a folate-maytansinoid conjugate. Cancer Res 67(13):6376–6382. https://doi.org/10.1158/0008-5472.CAN-06-3894
Li J, Sausville EA, Klein PJ, Morgenstern D, Leamon CP, Messmann RA, LoRusso P (2009) Clinical pharmacokinetics and exposure-toxicity relationship of a folate-Vinca alkaloid conjugate EC145 in cancer patients. J Clin Pharmacol 49(12):1467–1476. https://doi.org/10.1177/0091270009339740
Guertin AD, O’Neil J, Stoeck A, Reddy JA, Cristescu R, Haines BB, Hinton MC, Dorton R, Bloomfield A, Nelson M, Vetzel M, Lejnine S, Nebozhyn M, Zhang T, Loboda A, Picard KL, Schmidt EV, Dussault I, Leamon CP (2016) High levels of expression of p-glycoprotein/multidrug resistance protein result in resistance to vintafolide. Mol Cancer Ther 15(8):1998–2008. https://doi.org/10.1158/1535-7163.MCT-15-0950
Shan L, Zhuo X, Zhang F, Dai Y, Zhu G, Yung BC, Fan W, Zhai K, Jacobson O, Kiesewetter DO, Ma Y, Gao G, Chen X (2018) A paclitaxel prodrug with bifunctional folate and albumin binding moieties for both passive and active targeted cancer therapy. Theranostics 8(7):2018–2030. https://doi.org/10.7150/thno.24382
Liu Z, Xiong M, Gong J, Zhang Y, Bai N, Luo Y, Li L, Wei Y, Liu Y, Tan X, Xiang R (2014) Legumain protease-activated TAT-liposome cargo for targeting tumours and their microenvironment. Nat Commun 5:4280. https://doi.org/10.1038/ncomms5280. https://www.nature.com/articles/ncomms5280#supplementary-information
Zhang Y, Huang F, Ren C, Yang L, Liu J, Cheng Z, Chu L, Liu J (2017) Targeted chemo-photodynamic combination platform based on the DOX prodrug nanoparticles for enhanced cancer therapy. ACS Appl Mater Interfaces 9(15):13016–13028. https://doi.org/10.1021/acsami.7b00927
Regina A, Demeule M, Che C, Lavallee I, Poirier J, Gabathuler R, Beliveau R, Castaigne JP (2008) Antitumour activity of ANG1005, a conjugate between paclitaxel and the new brain delivery vector Angiopep-2. Br J Pharmacol 155(2):185–197. https://doi.org/10.1038/bjp.2008.260
Reddy JA, Dorton R, Bloomfield A, Nelson M, Dircksen C, Vetzel M, Kleindl P, Santhapuram H, Vlahov IR, Leamon CP (2018) Pre-clinical evaluation of EC1456, a folate-tubulysin anti-cancer therapeutic. Sci Rep 8(1):8943. https://doi.org/10.1038/s41598-018-27320-5
Funding
This review work was not supported by any grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Delahousse, J., Skarbek, C. & Paci, A. Prodrugs as drug delivery system in oncology. Cancer Chemother Pharmacol 84, 937–958 (2019). https://doi.org/10.1007/s00280-019-03906-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03906-2