Abstract
Purpose
High-dose methotrexate (HD-MTX) is widely used in the treatment of non-Hodgkin lymphoma (NHL), but the pharmacokinetic properties of HD-MTX in Chinese adult patients with NHL have not yet been established through an approach that integrates genetic covariates. The purposes of this study were to identify both physiological and pharmacogenomic covariates that can explain the inter- and intraindividual pharmacokinetic variability of MTX in Chinese adult patients with NHL and to explore a new sampling strategy for predicting delayed MTX elimination.
Methods
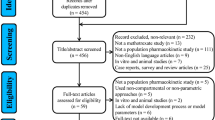
A total of 852 MTX concentrations from 91 adult patients with NHL were analyzed using the nonlinear mixed-effects modeling method. FPGS, GGH, SLCO1B1, ABCB1 and MTHFR were genotyped using the Sequenom MassARRAY technology platform and were screened as covariates. The ability of different sampling strategies to predict the MTX concentration at 72 h was assessed through maximum a posteriori Bayesian forecasting using a validation dataset (18 patients).
Results
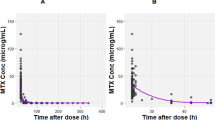
A two-compartment model adequately described the data, and the estimated mean MTX clearance (CL) was 6.03 L/h (9%). Creatinine clearance (CrCL) was identified as a covariate for CL, whereas the intercompartmental clearance (Q) was significantly affected by the body surface area (BSA). However, none of the genotypes exerted a significant effect on the pharmacokinetic properties of MTX. The percentage of patients with concentrations below 0.2 µmol/L at 72 h decreased from 65.6 to 42.6% when the CrCL decreased from 90 to 60 ml/min/1.73 m2 with a scheduled dosing of 3 g/m2, and the same trend was observed with dose regimens of 1 g/m2 and 2 g/m2. Bayesian forecasting using the MTX concentrations at 24 and 42 h provided the best predictive performance for estimating the MTX concentration at 72 h after dosing.
Conclusions
The MTX population pharmacokinetic model developed in this study might provide useful information for establishing personalized therapy involving MTX for the treatment of adult patients with NHL.











Similar content being viewed by others
References
Reiter A, Schrappe M, Tiemann M, Ludwig WD, Yakisan E, Zimmermann M, Mann G, Chott A, Ebell W, Klingebiel T, Graf N, Kremens B, Muller-Weihrich S, Pluss HJ, Zintl F, Henze G, Riehm H (1999) Improved treatment results in childhood B-cell neoplasms with tailored intensification of therapy: a report of the Berlin-Frankfurt-Munster Group Trial NHL-BFM 90. Blood 94(10):3294–3306
Maia MB, Saivin S, Chatelut E, Malmary MF, Houin G (1996) In vitro and in vivo protein binding of methotrexate assessed by microdialysis. Int J Clin Pharmacol Ther 34(8):335–341
Fotoohi AK, Albertioni F (2008) Mechanisms of antifolate resistance and methotrexate efficacy in leukemia cells. Leuk Lymphoma 49(3):410–426. https://doi.org/10.1080/10428190701824569
Hospira (2017) Label for methotrexate injection. Lake Forest, IL; Hospira; 2011. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/011719s117lbl.pdf. Accessed 29 Dec 2019
Takatori R, Takahashi KA, Tokunaga D, Hojo T, Fujioka M, Asano T, Hirata T, Kawahito Y, Satomi Y, Nishino H, Tanaka T, Hirota Y, Kubo T (2006) ABCB1 C3435T polymorphism influences methotrexate sensitivity in rheumatoid arthritis patients. Clin Exp Rheumatol 24(5):546–554
Trevino LR, Shimasaki N, Yang W, Panetta JC, Cheng C, Pei D, Chan D, Sparreboom A, Giacomini KM, Pui CH, Evans WE, Relling MV (2009) Germline genetic variation in an organic anion transporter polypeptide associated with methotrexate pharmacokinetics and clinical effects. J Clin Oncol 27(35):5972–5978. https://doi.org/10.1200/JCO.2008.20.4156
Bleyer WA (1978) The clinical pharmacology of methotrexate: new applications of an old drug. Cancer 41(1):36–51
Sterba J, Valik D, Bajciova V, Kadlecova V, Gregorova V, Mendelova D (2005) High-dose methotrexate and/or leucovorin rescue for the treatment of children with lymphoblastic malignancies: do we really know why, when and how? Neoplasma 52(6):456–463
Evans WE, Crom WR, Abromowitch M, Dodge R, Look AT, Bowman WP, George SL, Pui CH (1986) Clinical pharmacodynamics of high-dose methotrexate in acute lymphocytic leukemia. Identification of a relation between concentration and effect. N Engl J Med 314(8):471–477. https://doi.org/10.1056/NEJM198602203140803
Suthandiram S, Gan GG, Zain SM, Bee PC, Lian LH, Chang KM, Ong TC, Mohamed Z (2014) Effect of polymorphisms within methotrexate pathway genes on methotrexate toxicity and plasma levels in adults with hematological malignancies. Pharmacogenomics 15(11):1479–1494. https://doi.org/10.2217/pgs.14.97
Radtke S, Zolk O, Renner B, Paulides M, Zimmermann M, Moricke A, Stanulla M, Schrappe M, Langer T (2013) Germline genetic variations in methotrexate candidate genes are associated with pharmacokinetics, toxicity, and outcome in childhood acute lymphoblastic leukemia. Blood 121(26):5145–5153. https://doi.org/10.1182/blood-2013-01-480335
Chen Y, Shen Z (2015) Gene polymorphisms in the folate metabolism and their association with MTX-related adverse events in the treatment of ALL. Tumour Biol 36(7):4913–4921. https://doi.org/10.1007/s13277-015-3602-0
Lopez-Lopez E, Martin-Guerrero I, Ballesteros J, Garcia-Orad A (2013) A systematic review and meta-analysis of MTHFR polymorphisms in methotrexate toxicity prediction in pediatric acute lymphoblastic leukemia. Pharmacogenomics J 13(6):498–506. https://doi.org/10.1038/tpj.2012.44
Zhang HN, He XL, Wang C, Wang Y, Chen YJ, Li JX, Niu CH, Gao P (2014) Impact of SLCO1B1 521T %3e C variant on leucovorin rescue and risk of relapse in childhood acute lymphoblastic leukemia treated with high-dose methotrexate. Pediatr Blood Cancer 61(12):2203–2207. https://doi.org/10.1002/pbc.25191
Csordas K, Lautner-Csorba O, Semsei AF, Harnos A, Hegyi M, Erdelyi DJ, Eipel OT, Szalai C, Kovacs GT (2014) Associations of novel genetic variations in the folate-related and ARID5B genes with the pharmacokinetics and toxicity of high-dose methotrexate in paediatric acute lymphoblastic leukaemia. Br J Haematol 166(3):410–420. https://doi.org/10.1111/bjh.12886
Lima A, Bernardes M, Azevedo R, Monteiro J, Sousa H, Medeiros R, Seabra V (2014) SLC19A1, SLC46A1 and SLCO1B1 polymorphisms as predictors of methotrexate-related toxicity in Portuguese rheumatoid arthritis patients. Toxicol Sci 142(1):196–209. https://doi.org/10.1093/toxsci/kfu162
Avivi I, Zuckerman T, Krivoy N, Efrati E (2014) Genetic polymorphisms predicting methotrexate blood levels and toxicity in adult non-Hodgkin lymphoma. Leuk Lymphoma 55(3):565–570. https://doi.org/10.3109/10428194.2013.789506
Lopez-Lopez E, Martin-Guerrero I, Ballesteros J, Pinan MA, Garcia-Miguel P, Navajas A, Garcia-Orad A (2011) Polymorphisms of the SLCO1B1 gene predict methotrexate-related toxicity in childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 57(4):612–619. https://doi.org/10.1002/pbc.23074
Li J, Wang XR, Zhai XW, Wang HS, Qian XW, Miao H, Zhu XH (2015) Association of SLCO1B1 gene polymorphisms with toxicity response of high dose methotrexate chemotherapy in childhood acute lymphoblastic leukemia. Int J Clin Exp Med 8(4):6109–6113
Yasuda SU, Zhang L, Huang SM (2008) The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin Pharmacol Ther 84(3):417–423. https://doi.org/10.1038/clpt.2008.141
Faganel Kotnik B, Grabnar I, Bohanec Grabar P, Dolzan V, Jazbec J (2011) Association of genetic polymorphism in the folate metabolic pathway with methotrexate pharmacokinetics and toxicity in childhood acute lymphoblastic leukaemia and malignant lymphoma. Eur J Clin Pharmacol 67(10):993–1006. https://doi.org/10.1007/s00228-011-1046-z
Kim IW, Yun HY, Choi B, Han N, Park SY, Lee ES, Oh JM (2012) ABCB1 C3435T genetic polymorphism on population pharmacokinetics of methotrexate after hematopoietic stem cell transplantation in Korean patients: a prospective analysis. Clin Ther 34(8):1816–1826. https://doi.org/10.1016/j.clinthera.2012.06.022
Lui G, Treluyer JM, Fresneau B, Piperno-Neumann S, Gaspar N, Corradini N, Gentet JC, Marec Berard P, Laurence V, Schneider P, Entz-Werle N, Pacquement H, Millot F, Taque S, Freycon C, Lervat C, Le Deley MC, Mahier Ait Oukhatar C, Brugieres L, Le Teuff G, Bouazza N, Sarcoma Group of U (2018) A pharmacokinetic and pharmacogenetic analysis of osteosarcoma patients treated with high-dose methotrexate: data from the OS2006/sarcoma-09 trial. J Clin Pharmacol 58(12):1541–1549. https://doi.org/10.1002/jcph.1252
Cheah CY, Herbert KE, O'Rourke K, Kennedy GA, George A, Fedele PL, Gilbertson M, Tan SY, Ritchie DS, Opat SS, Prince HM, Dickinson M, Burbury K, Wolf M, Januszewicz EH, Tam CS, Westerman DA, Carney DA, Harrison SJ, Seymour JF (2014) A multicentre retrospective comparison of central nervous system prophylaxis strategies among patients with high-risk diffuse large B-cell lymphoma. Br J Cancer 111(6):1072–1079. https://doi.org/10.1038/bjc.2014.405
Hoang-Xuan K, Bessell E, Bromberg J, Hottinger AF, Preusser M, Ruda R, Schlegel U, Siegal T, Soussain C, Abacioglu U, Cassoux N, Deckert M, Dirven CM, Ferreri AJ, Graus F, Henriksson R, Herrlinger U, Taphoorn M, Soffietti R, Weller M, European Association for Neuro-Oncology Task Force on Primary CNSL (2015) Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: guidelines from the European Association for Neuro-Oncology. Lancet Oncol 16(7):e322–e332. https://doi.org/10.1016/S1470-2045(15)00076-5
Woessmann W, Seidemann K, Mann G, Zimmermann M, Burkhardt B, Oschlies I, Ludwig WD, Klingebiel T, Graf N, Gruhn B, Juergens H, Niggli F, Parwaresch R, Gadner H, Riehm H, Schrappe M, Reiter A, Group BFM (2005) The impact of the methotrexate administration schedule and dose in the treatment of children and adolescents with B-cell neoplasms: a report of the BFM Group Study NHL-BFM95. Blood 105(3):948–958. https://doi.org/10.1182/blood-2004-03-0973
Moricke A, Reiter A, Zimmermann M, Gadner H, Stanulla M, Dordelmann M, Loning L, Beier R, Ludwig WD, Ratei R, Harbott J, Boos J, Mann G, Niggli F, Feldges A, Henze G, Welte K, Beck JD, Klingebiel T, Niemeyer C, Zintl F, Bode U, Urban C, Wehinger H, Niethammer D, Riehm H, Schrappe M, German-Austrian-Swiss ALLBFMSG (2008) Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood 111(9):4477–4489. https://doi.org/10.1182/blood-2007-09-112920
Jaccard A, Gachard N, Marin B, Rogez S, Audrain M, Suarez F, Tilly H, Morschhauser F, Thieblemont C, Ysebaert L, Devidas A, Petit B, de Leval L, Gaulard P, Feuillard J, Bordessoule D, Hermine O, Gela IG (2011) Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood 117(6):1834–1839. https://doi.org/10.1182/blood-2010-09-307454
Dupuis C, Mercier C, Yang C, Monjanel-Mouterde S, Ciccolini J, Fanciullino R, Pourroy B, Deville JL, Duffaud F, Bagarry-Liegey D, Durand A, Iliadis A, Favre R (2008) High-dose methotrexate in adults with osteosarcoma: a population pharmacokinetics study and validation of a new limited sampling strategy. Anticancer Drugs 19(3):267–273
Keizer RJ, Karlsson MO, Hooker A (2013) Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacomet Syst Pharmacol 2:e50. https://doi.org/10.1038/psp.2013.24
Karlsson MO, Savic RM (2007) Diagnosing model diagnostics. Clin Pharmacol Ther 82(1):17–20. https://doi.org/10.1038/sj.clpt.6100241
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
Yafune A, Ishiguro M (1999) Bootstrap approach for constructing confidence intervals for population pharmacokinetic parameters. I: a use of bootstrap standard error. Stat Med 18(5):581–599
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO (2011) Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13(2):143–151. https://doi.org/10.1208/s12248-011-9255-z
Martinez D, Muhrez K, Woillard JB, Berthelot A, Gyan E, Choquet S, Andres CR, Marquet P, Barin-Le Guellec C (2018) Endogenous metabolites-mediated communication between OAT1/OAT3 and OATP1B1 may explain the association between SLCO1B1 SNPs and methotrexate toxicity. Clin Pharmacol Ther 104(4):687–698. https://doi.org/10.1002/cpt.1008
Woillard JB, Debord J, Benz-de-Bretagne I, Saint-Marcoux F, Turlure P, Girault S, Abraham J, Choquet S, Marquet P, Barin-Le Guellec C (2017) A time-dependent model describes methotrexate elimination and supports dynamic modification of MRP2/ABCC2 activity. Ther Drug Monit 39(2):145–156. https://doi.org/10.1097/FTD.0000000000000381
Faltaos DW, Hulot JS, Urien S, Morel V, Kaloshi G, Fernandez C, Xuan KH, Leblond V, Lechat P (2006) Population pharmacokinetic study of methotrexate in patients with lymphoid malignancy. Cancer Chemother Pharmacol 58(5):626–633. https://doi.org/10.1007/s00280-006-0202-0
Mei S, Li X, Jiang X, Yu K, Lin S, Zhao Z (2018) Population pharmacokinetics of high-dose methotrexate in patients with primary central nervous system lymphoma. J Pharm Sci 107(5):1454–1460. https://doi.org/10.1016/j.xphs.2018.01.004
Zhang W, Zhang Q, Tian X, Zhao H, Lu W, Zhen J, Niu X (2015) Population pharmacokinetics of high-dose methotrexate after intravenous administration in Chinese osteosarcoma patients from a single institution. Chin Med J (Engl) 128(1):111–118. https://doi.org/10.4103/0366-6999.147829
Simon N, Marsot A, Villard E, Choquet S, Khe HX, Zahr N, Lechat P, Leblond V, Hulot JS (2013) Impact of ABCC2 polymorphisms on high-dose methotrexate pharmacokinetics in patients with lymphoid malignancy. Pharmacogenomics J 13(6):507–513. https://doi.org/10.1038/tpj.2012.37
Aumente D, Buelga DS, Lukas JC, Gomez P, Torres A, Garcia MJ (2006) Population pharmacokinetics of high-dose methotrexate in children with acute lymphoblastic leukaemia. Clin Pharmacokinet 45(12):1227–1238. https://doi.org/10.2165/00003088-200645120-00007
Colom H, Farre R, Soy D, Peraire C, Cendros JM, Pardo N, Torrent M, Domenech J, Mangues MA (2009) Population pharmacokinetics of high-dose methotrexate after intravenous administration in pediatric patients with osteosarcoma. Ther Drug Monit 31(1):76–85. https://doi.org/10.1097/FTD.0b013e3181945624
Min Y, Qiang F, Peng L, Zhu Z (2009) High dose methotrexate population pharmacokinetics and Bayesian estimation in patients with lymphoid malignancy. Biopharm Drug Dispos 30(8):437–447. https://doi.org/10.1002/bdd.678
Odoul F, Le Guellec C, Lamagnere JP, Breilh D, Saux MC, Paintaud G, Autret-Leca E (1999) Prediction of methotrexate elimination after high dose infusion in children with acute lymphoblastic leukaemia using a population pharmacokinetic approach. Fundam Clin Pharmacol 13(5):595–604
Plard C, Bressolle F, Fakhoury M, Zhang D, Yacouben K, Rieutord A, Jacqz-Aigrain E (2007) A limited sampling strategy to estimate individual pharmacokinetic parameters of methotrexate in children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol 60(4):609–620. https://doi.org/10.1007/s00280-006-0394-3
Funding
This study was supported by the Fujian Provincial Health Technology Project (No: 2018-ZQN-18), the Natural Science Foundation of Fujian Province (No: 2016J01509), and the Science and Technology Program of Fuiian Province, China (No: 2018Y2003).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, L., Wu, H., de Winter, B.C.M. et al. Pharmacokinetics and pharmacogenetics of high-dose methotrexate in Chinese adult patients with non-Hodgkin lymphoma: a population analysis. Cancer Chemother Pharmacol 85, 881–897 (2020). https://doi.org/10.1007/s00280-020-04058-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-020-04058-4