Abstract
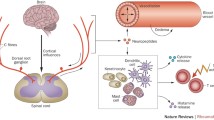
Fibromyalgia is a high impact chronic pain disorder with a well-defined and robust clinical phenotype. Key features include widespread pain and tenderness, high levels of sleep disturbance, fatigue, cognitive dysfunction and emotional distress. Abnormal processing of pain and other sensory input occurs in the brain, spinal cord and periphery and is related to the processes of central and peripheral sensitization. As such, fibromyalgia is deemed to be one of the central sensitivity syndromes. There is increasing evidence of neurogenically derived inflammatory mechanisms occurring in the peripheral tissues, spinal cord and brain in fibromyalgia. These involve a variety of neuropeptides, chemokines and cytokines with activation of both the innate and adaptive immune systems. This process results in several of the peripheral clinical features of fibromyalgia, such as swelling and dysesthesia, and may influence central symptoms, such as fatigue and changes in cognition. In turn, emotional and stress-related physiological mechanisms are seen as upstream drivers of neurogenic inflammation in fibromyalgia.
Similar content being viewed by others
References
Branco JC, Bannwarth B, Failde I, Abello Carbonell J, Blotman F, Spaeth M, Saraiva F, Nacci F, Thomas E, Caubere JP, Le Lay K, Taieb C, Matucci-Cerinic M (2010) Prevalence of fibromyalgia: a survey in five European countries. Semin Arthritis Rheum 39(6):448–453. https://doi.org/10.1016/j.semarthrit.2008.12.003
Gerdle B, Bjork J, Coster L, Henriksson K, Henriksson C, Bengtsson A (2008) Prevalence of widespread pain and associations with work status: a population study. BMC Musculoskelet Disord 9:102. https://doi.org/10.1186/1471-2474-9-102
Clauw DJ (2014) Fibromyalgia: a clinical review. JAMA 311(15):1547–1555. https://doi.org/10.1001/jama.2014.3266
Theoharides TC, Tsilioni I, Arbetman L, Panagiotidou S, Stewart JM, Gleason RM, Russell IJ (2015) Fibromyalgia syndrome in need of effective treatments. J Pharmacol Exp Ther 355(2):255–263. https://doi.org/10.1124/jpet.115.227298
Haviland MG, Morton KR, Oda K, Fraser GE (2010) Traumatic experiences, major life stressors, and self-reporting a physician-given fibromyalgia diagnosis. Psychiatry Res 177(3):335–341. https://doi.org/10.1016/j.psychres.2009.08.017
Van Houdenhove B, Egle U, Luyten P (2005) The role of life stress in fibromyalgia. Curr Rheumatol Rep 7(5):365–370
Helme RD, Littlejohn GO, Weinstein C (1987) Neurogenic flare responses in chronic rheumatic pain syndromes. Clin Exp Neurol 23:91–94
Clauw DJ, Arnold LM, McCarberg BH, FibroCollaborative (2011) The science of fibromyalgia. Mayo Clin Proc 86(9):907–911. https://doi.org/10.4065/mcp.2011.0206
Thiagarajah AT, Guymer E, Leech M, Littlejohn G (2014) The relationship between fibromyalgia, stress and depression. Int J Clin Rheum 9(4):371–384
Littlejohn G (2014) Fibromyalgia: honing fibromyalgia diagnosis. Nat Rev Rheumatol 10(5):267–269. https://doi.org/10.1038/nrrheum.2014.48
Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Michael Franklin C, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, Mccain GA, John Reynolds W, Romano TJ, Jon Russell I, Sheon RP (1990) The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum 33(2):160–172
Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB (2010) The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res 62(5):600–610. https://doi.org/10.1002/acr.20140
Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Hauser W, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB (2011) Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR preliminary diagnostic criteria for fibromyalgia. J Rheumatol 38(6):1113–1122. https://doi.org/10.3899/jrheum.100594
Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Hauser W, Katz RL, Mease PJ, Russell AS, Russell IJ, Walitt B (2016) 2016 revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum 46(3):319–329. https://doi.org/10.1016/j.semarthrit.2016.08.012
Yunus MB (2015) Editorial review: an update on central sensitivity syndromes and the issues of nosology and psychobiology. Curr Rheumatol Rev 11(2):70–85
Yunus MB (2007) Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum 36(6):339–356. https://doi.org/10.1016/j.semarthrit.2006.12.009
Napadow V, Kim J, Clauw DJ, Harris RE (2012) Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum 64(7):2398–2403. https://doi.org/10.1002/art.34412
Jensen KB, Loitoile R, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Mainguy Y, Vitton O, Gracely RH, Gollub R, Ingvar M, Kong J (2012) Patients with fibromyalgia display less functional connectivity in the brain’s pain inhibitory network. Mol Pain 8:32. https://doi.org/10.1186/1744-8069-8-32
Harris RE, Sundgren PC, Craig AD, Kirshenbaum E, Sen A, Napadow V, Clauw DJ (2009) Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum 60(10):3146–3152. https://doi.org/10.1002/art.24849
Vaeroy H, Helle R, Forre O, Kass E, Terenius L (1988) Elevated CSF levels of substance P and high incidence of Raynaud phenomenon in patients with fibromyalgia: new features for diagnosis. Pain 32(1):21–26
Russell IJ, Orr MD, Littman B, Vipraio GA, Alboukrek D, Michalek JE, Lopez Y, MacKillip F (1994) Elevated cerebrospinal fluid levels of substance P in patients with the fibromyalgia syndrome. Arthritis Rheum 37(11):1593–1601
Giovengo SL, Russell IJ, Larson AA (1999) Increased concentrations of nerve growth factor in cerebrospinal fluid of patients with fibromyalgia. J Rheumatol 26(7):1564–1569
Sarchielli P, Mancini ML, Floridi A, Coppola F, Rossi C, Nardi K, Acciarresi M, Pini LA, Calabresi P (2007) Increased levels of neurotrophins are not specific for chronic migraine: evidence from primary fibromyalgia syndrome. J Pain 8(9):737–745. https://doi.org/10.1016/j.jpain.2007.05.002
Vaeroy H, Sakurada T, Forre O, Kass E, Terenius L (1989) Modulation of pain in fibromyalgia (fibrositis syndrome): cerebrospinal fluid (CSF) investigation of pain related neuropeptides with special reference to calcitonin gene related peptide (CGRP). J Rheumatol Suppl 19:94–97
Reynolds WJ, Chiu B, Inman RD (1988) Plasma substance P levels in fibrositis. J Rheumatol 15(12):1802–1803
Tsilioni I, Russell IJ, Stewart JM, Gleason RM, Theoharides TC (2016) Neuropeptides CRH, SP, HK-1, and inflammatory cytokines IL-6 and TNF are increased in serum of patients with fibromyalgia syndrome, implicating mast cells. J Pharmacol Exp Ther 356(3):664–672. https://doi.org/10.1124/jpet.115.230060
Kadetoff D, Lampa J, Westman M, Andersson M, Kosek E (2012) Evidence of central inflammation in fibromyalgia-increased cerebrospinal fluid interleukin-8 levels. J Neuroimmunol 242(1–2):33–38. https://doi.org/10.1016/j.jneuroim.2011.10.013
Kosek E, Martinsen S, Gerdle B, Mannerkorpi K, Lofgren M, Bileviciute-Ljungar I, Fransson P, Schalling M, Ingvar M, Ernberg M, Jensen KB (2016) The translocator protein gene is associated with symptom severity and cerebral pain processing in fibromyalgia. Brain Behav Immun 58:218–227. https://doi.org/10.1016/j.bbi.2016.07.150
Backryd E, Tanum L, Lind AL, Larsson A, Gordh T (2017) Evidence of both systemic inflammation and neuroinflammation in fibromyalgia patients, as assessed by a multiplex protein panel applied to the cerebrospinal fluid and to plasma. J Pain Res 10:515–525. https://doi.org/10.2147/JPR.S128508
Rea K, Dinan TG, Cryan JF (2016) The microbiome: a key regulator of stress and neuroinflammation. Neurobiol Stress 4:23–33. https://doi.org/10.1016/j.ynstr.2016.03.001
Lyon P, Cohen M, Quintner J (2011) An evolutionary stress-response hypothesis for chronic widespread pain (fibromyalgia syndrome). Pain Med 12(8):1167–1178. https://doi.org/10.1111/j.1526-4637.2011.01168.x
Geracioti TD Jr, Carpenter LL, Owens MJ, Baker DG, Ekhator NN, Horn PS, Strawn JR, Sanacora G, Kinkead B, Price LH, Nemeroff CB (2006) Elevated cerebrospinal fluid substance p concentrations in posttraumatic stress disorder and major depression. Am J Psychiatry 163(4):637–643. https://doi.org/10.1176/appi.ajp.163.4.637
Xanthos DN, Sandkuhler J (2014) Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci 15(1):43–53. https://doi.org/10.1038/nrn3617
Garate I, Garcia-Bueno B, Madrigal JL, Bravo L, Berrocoso E, Caso JR, Mico JA, Leza JC (2011) Origin and consequences of brain toll-like receptor 4 pathway stimulation in an experimental model of depression. J Neuroinflammation 8:151. https://doi.org/10.1186/1742-2094-8-151
Rosenkranz MA, Davidson RJ, Maccoon DG, Sheridan JF, Kalin NH, Lutz A (2013) A comparison of mindfulness-based stress reduction and an active control in modulation of neurogenic inflammation. Brain Behav Immun 27(1):174–184. https://doi.org/10.1016/j.bbi.2012.10.013
Lentz MJ, Landis CA, Rothermel J, Shaver JL (1999) Effects of selective slow wave sleep disruption on musculoskeletal pain and fatigue in middle aged women. J Rheumatol 26(7):1586–1592
Zhu B, Dong Y, Xu Z, Gompf HS, Ward SA, Xue Z, Miao C, Zhang Y, Chamberlin NL, Xie Z (2012) Sleep disturbance induces neuroinflammation and impairment of learning and memory. Neurobiol Dis 48(3):348–355. https://doi.org/10.1016/j.nbd.2012.06.022
Malin K, Littlejohn GO (2016) Psychological factors mediate key symptoms of fibromyalgia through their influence on stress. Clin Rheumatol 35(9):2353–2357. https://doi.org/10.1007/s10067-016-3315-9
Milligan ED, Watkins LR (2009) Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci 10(1):23–36. https://doi.org/10.1038/nrn2533
Watkins LR, Maier SF (2005) Immune regulation of central nervous system functions: from sickness responses to pathological pain. J Intern Med 257(2):139–155. https://doi.org/10.1111/j.1365-2796.2004.01443.x
Desmeules JA, Cedraschi C, Rapiti E, Baumgartner E, Finckh A, Cohen P, Dayer P, Vischer TL (2003) Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum 48(5):1420–1429. https://doi.org/10.1002/art.10893
Cagnie B, Coppieters I, Denecker S, Six J, Danneels L, Meeus M (2014) Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Semin Arthritis Rheum 44(1):68–75. https://doi.org/10.1016/j.semarthrit.2014.01.001
Sluka KA, Clauw DJ (2016) Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience 338:114–129. https://doi.org/10.1016/j.neuroscience.2016.06.006
Kosek E, Hansson P (1997) Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain 70(1):41–51
Julien N, Goffaux P, Arsenault P, Marchand S (2005) Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain 114(1–2):295–302. https://doi.org/10.1016/j.pain.2004.12.032
Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK (2007) Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci 27(37):10000–10006. https://doi.org/10.1523/JNEUROSCI.2849-07.2007
Woolf CJ (2011) Central sensitization: implications for the diagnosis and treatment of pain. Pain 152(3 Suppl):S2–S15. https://doi.org/10.1016/j.pain.2010.09.030
Roche S, Gabelle A, Lehmann S (2008) Clinical proteomics of the cerebrospinal fluid: towards the discovery of new biomarkers. Proteomics Clin Appl 2(3):428–436. https://doi.org/10.1002/prca.200780040
Lewis T (1927) The blood vessels of the human skin and their responses. Shaw and Sons, London
Wallengren J, Moller H (1986) The effect of capsaicin on some experimental inflammations in human skin. Acta Derm Venereol 66(5):375–380
Holzer P (1998) Neurogenic vasodilatation and plasma leakage in the skin. Gen Pharmacol 30(1):5–11
Schmelz M, Michael K, Weidner C, Schmidt R, Torebjork HE, Handwerker HO (2000) Which nerve fibers mediate the axon reflex flare in human skin? Neuroreport 11(3):645–648
Chiu IM, von Hehn CA, Woolf CJ (2012) Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci 15(8):1063–1067. https://doi.org/10.1038/nn.3144
Littlejohn GO, Weinstein C, Helme RD (1987) Increased neurogenic inflammation in fibrositis syndrome. J Rheumatol 14(5):1022–1025
Yunus M, Masi AT, Calabro JJ, Miller KA, Feigenbaum SL (1981) Primary fibromyalgia (fibrositis): clinical study of 50 patients with matched normal controls. Semin Arthritis Rheum 11(1):151–171
Hauser W, Hayo S, Biewer W, Gesmann M, Kuhn-Becker H, Petzke F, von Wilmoswky H, Langhorst J (2010) Diagnosis of fibromyalgia syndrome—a comparison of Association of the Medical Scientific Societies in Germany, survey, and American College of Rheumatology criteria. Clin J Pain 26(6):505–511. https://doi.org/10.1097/AJP.0b013e3181d92a6c
Littlejohn G, Granges G (1995) The relationship between vertebral dysfunction and clinical features of fibromyalgia syndrome. J Orthopedic Rheum 8:97–105
Pay S, Calguneri M, Caliskaner Z, Dinc A, Apras S, Ertenli I, Kiraz S, Cobankara V (2000) Evaluation of vascular injury with proinflammatory cytokines, thrombomodulin and fibronectin in patients with primary fibromyalgia. Nagoya J Med Sci 63(3–4):115–122
Caro XJ (1984) Immunofluorescent detection of IgG at the dermal-epidermal junction in patients with apparent primary fibrositis syndrome. Arthritis Rheum 27(10):1174–1179
Birklein F, Schmelz M (2008) Neuropeptides, neurogenic inflammation and complex regional pain syndrome (CRPS). Neurosci Lett 437(3):199–202. https://doi.org/10.1016/j.neulet.2008.03.081
Blanco I, Beritze N, Arguelles M, Carcaba V, Fernandez F, Janciauskiene S, Oikonomopoulou K, de Serres FJ, Fernandez-Bustillo E, Hollenberg MD (2010) Abnormal overexpression of mastocytes in skin biopsies of fibromyalgia patients. Clin Rheumatol 29(12):1403–1412. https://doi.org/10.1007/s10067-010-1474-7
Enestrom S, Bengtson A, Lindstrom F, Johan K (1990) Attachment of IgG to dermal extracellular matrix in patients with fibromyalgia. Clin Exp Rheumatol 8(2):127–135
Enestrom S, Bengtsson A, Frodin T (1997) Dermal IgG deposits and increase of mast cells in patients with fibromyalgia—relevant findings or epiphenomena? Scand J Rheumatol 26(4):308–313
Caro XJ, Wolfe F, Johnston WH, Smith AL (1986) A controlled and blinded study of immunoreactant deposition at the dermal-epidermal junction of patients with primary fibrositis syndrome. J Rheumatol 13(6):1086–1092
Caro XJ (1986) Immunofluorescent studies of skin in primary fibrositis syndrome. Am J Med 81(3A):43–49
Rodriguez-Pinto I, Agmon-Levin N, Howard A, Shoenfeld Y (2014) Fibromyalgia and cytokines. Immunol Lett 161(2):200–203. https://doi.org/10.1016/j.imlet.2014.01.009
Generaal E, Vogelzangs N, Macfarlane GJ, Geenen R, Smit JH, Dekker J, Penninx BW (2014) Basal inflammation and innate immune response in chronic multisite musculoskeletal pain. Pain 155(8):1605–1612. https://doi.org/10.1016/j.pain.2014.05.007
Uceyler N, Hauser W, Sommer C (2011) Systematic review with meta-analysis: cytokines in fibromyalgia syndrome. BMC Musculoskelet Disord 12:245. https://doi.org/10.1186/1471-2474-12-245
Mendieta D, De la Cruz-Aguilera DL, Barrera-Villalpando MI, Becerril-Villanueva E, Arreola R, Hernandez-Ferreira E, Perez-Tapia SM, Perez-Sanchez G, Garces-Alvarez ME, Aguirre-Cruz L, Velasco-Velazquez MA, Pavon L (2016) IL-8 and IL-6 primarily mediate the inflammatory response in fibromyalgia patients. J Neuroimmunol 290:22–25. https://doi.org/10.1016/j.jneuroim.2015.11.011
Gerdle B, Ghafouri B, Ghafouri N, Backryd E, Gordh T (2017) Signs of ongoing inflammation in female patients with chronic widespread pain: a multivariate, explorative, cross-sectional study of blood samples. Medicine 96(9):e6130. https://doi.org/10.1097/MD.0000000000006130
Ranzolin A, Duarte AL, Bredemeier M, da Costa Neto CA, Ascoli BM, Wollenhaupt-Aguiar B, Kapczinski F, Xavier RM (2016) Evaluation of cytokines, oxidative stress markers and brain-derived neurotrophic factor in patients with fibromyalgia—a controlled cross-sectional study. Cytokine 84:25–28. https://doi.org/10.1016/j.cyto.2016.05.011
Littlejohn G (2015) Neurogenic neuroinflammation in fibromyalgia and complex regional pain syndrome. Nat Rev Rheumatol 11(11):639–648. https://doi.org/10.1038/nrrheum.2015.100
Behm FG, Gavin IM, Karpenko O, Lindgren V, Gaitonde S, Gashkoff PA, Gillis BS (2012) Unique immunologic patterns in fibromyalgia. BMC Clin Pathol 12:25. https://doi.org/10.1186/1472-6890-12-25
Christidis N, Ghafouri B, Larsson A, Palstam A, Mannerkorpi K, Bileviciute-Ljungar I, Lofgren M, Bjersing J, Kosek E, Gerdle B, Ernberg M (2015) Comparison of the levels of pro-inflammatory cytokines released in the vastus lateralis muscle of patients with fibromyalgia and healthy controls during contractions of the quadriceps muscle—a microdialysis study. PLoS One 10(12):e0143856. https://doi.org/10.1371/journal.pone.0143856
Heijmans-Antonissen C, Wesseldijk F, Munnikes RJ, Huygen FJ, van der Meijden P, Hop WC, Hooijkaas H, Zijlstra FJ (2006) Multiplex bead array assay for detection of 25 soluble cytokines in blister fluid of patients with complex regional pain syndrome type 1. Mediat Inflamm 2006(1):28398–28398. https://doi.org/10.1155/MI/2006/28398
Huygen FJ, De Bruijn AG, De Bruin MT, Groeneweg JG, Klein J, Zijlstra FJ (2002) Evidence for local inflammation in complex regional pain syndrome type 1. Mediat Inflamm 11(1):47–51. https://doi.org/10.1080/09629350210307
Munnikes RJ, Muis C, Boersma M, Heijmans-Antonissen C, Zijlstra FJ, Huygen FJ (2005) Intermediate stage complex regional pain syndrome type 1 is unrelated to proinflammatory cytokines. Mediat Inflamm 2005(6):366–372. https://doi.org/10.1155/MI.2005.366
Martinez-Martinez LA, Mora T, Vargas A, Fuentes-Iniestra M, Martinez-Lavin M (2014) Sympathetic nervous system dysfunction in fibromyalgia, chronic fatigue syndrome, irritable bowel syndrome, and interstitial cystitis: a review of case-control studies. J Clin Rheumatol 20(3):146–150. https://doi.org/10.1097/RHU.0000000000000089
Lerma C, Martinez A, Ruiz N, Vargas A, Infante O, Martinez-Lavin M (2011) Nocturnal heart rate variability parameters as potential fibromyalgia biomarker: correlation with symptoms severity. Arthritis Res Ther 13(6):R185. https://doi.org/10.1186/ar3513
Lerma C, Martinez-Martinez LA, Ruiz N, Vargas A, Infante O, Martinez-Lavin M (2016) Fibromyalgia beyond reductionism. Heart rhythm fractal analysis to assess autonomic nervous system resilience. Scand J Rheumatol 45(2):151–157. https://doi.org/10.3109/03009742.2015.1055299
Dawson LF, Phillips JK, Finch PM, Inglis JJ, Drummond PD (2011) Expression of alpha1-adrenoceptors on peripheral nociceptive neurons. Neuroscience 175:300–314. https://doi.org/10.1016/j.neuroscience.2010.11.064
Maestroni GJ (2006) Sympathetic nervous system influence on the innate immune response. Ann N Y Acad Sci 1069:195–207. https://doi.org/10.1196/annals.1351.017
Kosek E, Altawil R, Kadetoff D, Finn A, Westman M, Le Maitre E, Andersson M, Jensen-Urstad M, Lampa J (2015) Evidence of different mediators of central inflammation in dysfunctional and inflammatory pain—interleukin-8 in fibromyalgia and interleukin-1 beta in rheumatoid arthritis. J Neuroimmunol 280:49–55. https://doi.org/10.1016/j.jneuroim.2015.02.002
Uceyler N, Zeller D, Kahn AK, Kewenig S, Kittel-Schneider S, Schmid A, Casanova-Molla J, Reiners K, Sommer C (2013) Small fibre pathology in patients with fibromyalgia syndrome. Brain 136(Pt 6):1857–1867. https://doi.org/10.1093/brain/awt053
de Tommaso M, Nolano M, Iannone F, Vecchio E, Ricci K, Lorenzo M, Delussi M, Girolamo F, Lavolpe V, Provitera V, Stancanelli A, Lapadula G, Livrea P (2014) Update on laser-evoked potential findings in fibromyalgia patients in light of clinical and skin biopsy features. J Neurol 261(3):461–472. https://doi.org/10.1007/s00415-013-7211-9
Giannoccaro MP, Donadio V, Incensi A, Avoni P, Liguori R (2014) Small nerve fiber involvement in patients referred for fibromyalgia. Muscle Nerve 49(5):757–759. https://doi.org/10.1002/mus.24156
Kosmidis ML, Koutsogeorgopoulou L, Alexopoulos H, Mamali I, Vlachoyiannopoulos PG, Voulgarelis M, Moutsopoulos HM, Tzioufas AG, Dalakas MC (2014) Reduction of Intraepidermal nerve fiber density (IENFD) in the skin biopsies of patients with fibromyalgia: a controlled study. J Neurol Sci 347(1–2):143–147. https://doi.org/10.1016/j.jns.2014.09.035
Oaklander AL, Herzog ZD, Downs HM, Klein MM (2013) Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain 154(11):2310–2316. https://doi.org/10.1016/j.pain.2013.06.001
Caro XJ, Winter EF (2015) The role and importance of small fiber neuropathy in fibromyalgia pain. Curr Pain Headache Rep 19(12):55. https://doi.org/10.1007/s11916-015-0527-7
Doppler K, Rittner HL, Deckart M, Sommer C (2015) Reduced dermal nerve fiber diameter in skin biopsies of patients with fibromyalgia. Pain 156(11):2319–2325. https://doi.org/10.1097/j.pain.0000000000000285
Caro XJ, Winter EF (2014) Evidence of abnormal epidermal nerve fiber density in fibromyalgia: clinical and immunologic implications. Arthritis Rheumatol 66(7):1945–1954. https://doi.org/10.1002/art.38662
Leinders M, Doppler K, Klein T, Deckart M, Rittner H, Sommer C, Uceyler N (2016) Increased cutaneous miR-let-7d expression correlates with small nerve fiber pathology in patients with fibromyalgia syndrome. Pain 157(11):2493–2503. https://doi.org/10.1097/j.pain.0000000000000668
Serra J, Collado A, Sola R, Antonelli F, Torres X, Salgueiro M, Quiles C, Bostock H (2014) Hyperexcitable C nociceptors in fibromyalgia. Ann Neurol 75(2):196–208. https://doi.org/10.1002/ana.24065
Kim SH, Kim DH, Oh DH, Clauw DJ (2008) Characteristic electron microscopic findings in the skin of patients with fibromyalgia: preliminary study. Clin Rheumatol 27(2):219–223. https://doi.org/10.1007/s10067-007-0739-2
Ramirez M, Martinez-Martinez LA, Hernandez-Quintela E, Velazco-Casapia J, Vargas A, Martinez-Lavin M (2015) Small fiber neuropathy in women with fibromyalgia. An in vivo assessment using corneal confocal bio-microscopy. Semin Arthritis Rheum 45(2):214–219. https://doi.org/10.1016/j.semarthrit.2015.03.003
Watson NF, Buchwald D, Goldberg J, Noonan C, Ellenbogen RG (2009) Neurologic signs and symptoms in fibromyalgia. Arthritis Rheum 60(9):2839–2844. https://doi.org/10.1002/art.24772
Harte S, Clauw D, Hayes JM, Feldman EL, St Charles IC, Watson CJ (2017) Reduced intraepidermal nerve fiber density after a sustained increase in insular glutamate: a proof-of-concept study examining the pathogenesis of small fiber pathology in fibromyalgia. Pain Rep 2(e590):1–6
Staud R, Nagel S, Robinson ME, Price DD (2009) Enhanced central pain processing of fibromyalgia patients is maintained by muscle afferent input: a randomized, double-blind, placebo-controlled study. Pain 145(1–2):96–104. https://doi.org/10.1016/j.pain.2009.05.020
Baron R, Hans G, Dickenson AH (2013) Peripheral input and its importance for central sensitization. Ann Neurol 74(5):630–636. https://doi.org/10.1002/ana.24017
Grover S, Srivastava A, Lee R, Tewari AK, Te AE (2011) Role of inflammation in bladder function and interstitial cystitis. Ther Adv Urol 3(1):19–33. https://doi.org/10.1177/1756287211398255
Malhotra R (2016) Understanding migraine: potential role of neurogenic inflammation. Ann Indian Acad Neurol 19(2):175–182. https://doi.org/10.4103/0972-2327.182302
Feng B, La JH, Schwartz ES, Gebhart GF (2012) Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Neural and neuro-immune mechanisms of visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 302(10):G1085–G1098. https://doi.org/10.1152/ajpgi.00542.2011
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is a contribution to the special issue on Neurogenic Inflammation - Guest Editors: Tony Yaksh and Anna Di Nardo
Rights and permissions
About this article
Cite this article
Littlejohn, G., Guymer, E. Neurogenic inflammation in fibromyalgia. Semin Immunopathol 40, 291–300 (2018). https://doi.org/10.1007/s00281-018-0672-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-018-0672-2