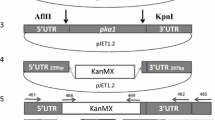
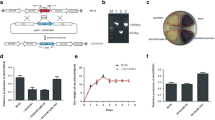
Abstract
Environmental glucose is an important regulator of biological processes, as it can launch different cell processes depending on its concentration. Thus, low glucose concentration can induce entry into quiescence, which ensures long-term viability for the cells or in other cases mycelial growth in the dimorphic species, which, in turn, provides the cells with fresh nutrients. Several genes, such as the genes of cAMP cascade, are involved in glucose sensing and response. Since this signal transduction pathway seemed to be an evolutionarily conserved process, we assumed that its genes were also conserved and preserved their functional homology. To obtain evidence, Schizosaccharomyces pombe rsv1 and its orthologous genes were investigated using in silico and experimental approaches. Our results supported that the Rsv1 zinc-finger transcription factors of Schizosaccharomyces japonicus and Schizosaccharomyces octosporus and the Candida albicans cas5p were really functional homologues of the S. pombe Rsv1. Namely, the homologous proteins were able to restore mutant phenotype of the S. pombe rsv1-deleted cells. Bioinformatic anaysis revealed that the most conserved parts of the proteins always contained the C2H2 domains and the complementation abilities of the counterpart genes were not uniform regarding the investigated features, which can be in connection with the conserved regions outside C2H2.



Similar content being viewed by others
References
Alfa C, Fantes P, Hyams J, Warbrich ME (eds) (1993) Experiments with fission yeast: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Balazs A, Batta G, Miklos I, Acs-Szabo L, Vazquez de Aldana CR, Sipiczki M (2012) Conserved regulators of the cell separation process in Schizosaccharomyces. Fungal Genet Biol 49(3):235–249
Bockmühl DP, Krishnamurthy S, Gerads M, Sonneborn A, Ernst JF (2001) Distinct and redundant roles of the two protein kinase A isoforms Tpk1p and Tpk2p in morphogenesis and growth of Candida albicans. Mol Microbiol 42(5):1243–1257
Brown V, Sexton JA, Johnston M (2006) A Glucose sensor in Candida albicans. Eukaryot Cell 5(10):1726–1737
Bruno VM, Kalachikov S, Subaran R, Nobile CJ, Kyratsous C, Mitchell AP (2006) Control of the c. albicans cell wall damage response by transcriptional regulator cas5. PLoS Pathog 2(3):e21
Buu LM, Chen YC (2014) Impact of glucose levels on expression of hypha-associated secreted aspartyl proteinases in Candida albicans. J Biomed Sci 21:22–31
Crooks GE, Hon G, Chandonia JM, Brenner SE (2004) WebLogo: a sequence logo generator. Genome Res 14:1188–1190
Cullen PJ, Sprague GF Jr (2000) Glucose depletion causes haploid invasive growth in yeast. PNAS 97(25):13619–13624
Giacometti R, Kronberg F, Biondi RM, Passeron S (2011) Candida albicans Tpk1p and Tpk2p isoforms differentially regulate pseudohyphal development, biofilm structure, cell aggregation and adhesins expression. Yeast 28:293–308
Gupta DR, Paul SK, Oowatari Y, Matsuo Y, Kawamukai M (2011) Complex Formation, Phosphorylation, and Localization of Protein Kinase A of Schizosaccharomyces pombe upon Glucose Starvation. Biosci Biotechnol Biochem 75(8):1456–1465
Hao Z, Furunobu A, Nagata A, Okayama H (1997) A zinc finger protein required for stationary phase viability in fission yeast. J Cell Sci 110:2557–2566
Heiland S, Radovanovic N, Hofer M, Winderickx J and Lichtenberg H (2000) Multiple Hexose Transporters of Schizosaccharomyces pombe J of Bact 182(8):2153–2162
Herman PK Stationary phase in yeast. Curr Opin Microbiol 5(6):602–7
Homann OR, Dea J, Noble SM, Johnson AD (2009) A Phenotypic Profile of the Candida albicans Regulatory Network. PLoS Genet 5(12):e1000783
Hoffman CS (2005) Except in every detail: comparing and contrasting G protein signaling in Saccharomyces cerevisiae and Schizosaccharomyces pombe. Eukaryot Cell 4:495–503
Hoffman CS (2005) Glucose sensing via the protein kinase A pathway in Schizosaccharomyces pombe. Biochem Soc Trans 33(01):257–260
Hudson DA, Sciascia QL, Sanders RJ, Norris GE, Edwards P J B, Sullivan PA, Farley PC (2004) Identification of the dialysable serum inducer of germ-tube formation in Candida albicans. Microbiology 150:3041–3049
Hrmová M, Drobnica L (1981) Induction of mycelial type of development in Candida albicans by low glucose concentration. Mycopathologia 76(2):83–96
Kraakman L, Lemaire K, Ma P, Teunissen A WRH, Donaton MCV, Van Dijck P, Winderickx J, de Winde JH, Thevelein JM (2000) The G protein-coupled receptor gpr1 is a nutrient sensor that regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Genetics 154(2):609–622
Lyne R, Burns G, Mata J, Penkett CJ, Rustici G, Chen D, Langford C, Vetrie D, Bähler J (2003) Whole-genome microarrays of fission yeast: characteristics, accuracy, reproducibility, and processing of array data. BMC Genomics 4:27–42
Liu J, Jia Y, Li J, Chu Z (2015) Transcriptional profiling analysis of individual kinase-deletion strains of fission yeast in response to nitrogen starvation. Mol Genet Genomics 290:1067–1083
Maidan MM, De Rop L, Serneels J, Exler S, Rupp S, Tournu H, Thevelein JM, Van Dijck P (2005) The G protein-coupled receptor Gpr1 and the G alpha protein Gpa2 act through the cAMP-protein kinase. A pathway to induce morphogenesis in Candida albicans. Mol Biol Cell 16(4):1971–1986
Mata J, Bahler J (2007) Transcriptional regulatory network for sexual differentiation in fission yeast. Genome Biol 8(10):R217
Maundrell K (1993) Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene 123:127–130
Marchler-Bauer A, Derbyshire MK, Gonzales NR, Lu S, Chitsaz F, Geer LY, Geer RC, He J, Gwadz M, Hurwitz DI, Lanczycki CJ, Lu F, Marchler GH, Song JS, Thanki N, Wang Z, Yamashita RA, Zhang D, Zheng C, Bryant SH (2015) CDD:NCBI’s conserved domain database. Nucleic Acids Res 43:D222–D226
Mitchison JM (1970) Physiological and cytological methods for Schizosaccharomyces pombe. Methods Cell Physiol 4:131–165
Miwa T, Takagi Y, Shinozaki M, Yun CW, Schell WA, Perfect JR, Kumagai H, Tamaki H (2004) Gpr1, a putative G-protein-coupled receptor, regulates morphogenesis and hypha formation in the pathogenic fungus Candida albicans. Eukaryot Cell 3(4):919–931
Ozcan S, Johnston M (1999) Function and regulation of yeast hexose transporters. Microbiol Mol Biol Rev 63(3):554–569
Pan X, Lei B, Zhou N, Feng B, Yao W, Zhao X, Yu Y, Lu H (2012) “Identification of novel genes involved in DNA damage response by screening a genome-wide Schizosaccharomyces pombe deletion library”. BMC Genomics 13:662
Papp L, Sipiczki M., Miklos I (2016) Expression pattern and phenotypic characterization of the mutant strain reveals target genes and processes regulated by pka1 in the dimorphic fission yeast Schizosaccharomyces japonicus. Curr Genet (in press) DOI:10.1007/s00294-016-0651-x
Pletnev P, Osterman I, Sergiev P, Bogdanov A, Dontsova O (2015) Survival guide: Escherichia coli in the stationary phase, Acta Naturae 7(4): 22–33
Prentice HL (1992) High efficiency transformation of Schizosaccharomyces pombe by electroporation. Nucleic Acids Res 20:621
Rhind et al (2011) “Comparative Functional Genomics of the Fission Yeasts”. Science 332(6032):930–936
Robertson LS, Fink GR (1998) The three yeast A kinases have specific signaling functions in pseudohyphal growth. Proc Natl Acad Sci USA 95(23):13783–13787
Sabina J, Brown V (2009) Glucose sensing network in Candida albicans: a sweet spot for fungal morphogenesis. Eukaryot Cell 8(9):1314–1320
Santangelo GM (2006) Glucose Signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 70(1):253–282
Sipiczki M, Ferenczy L (1977) Protoplast fusion of Schizosaccharomyces pombe auxotrophic mutants of identical mating-type. Mol Gen Genet 151:77–78
Sipiczki M, Takeo K, Grallert A (1998) Growth polarity transitions in adimorphic fission yeast. Microbiology 144(12):3475–3485
Wang Y, Pierce M, Schneper L, Güldal CG, Zhang X, Tavazoie S, Broach JR (2004) Ras and Gpa2 Mediate One Branch of a Redundant Glucose Signaling Pathway in Yeast. PLoS Biol 2(5):e128
Welton RM, Hoffman CS (2000) Glucose monitoring in fission yeast via the gpa2 G, the git5 G, and the git3 putative glucose receptor. Genetics 156:513–521
Yun CW, Tamaki H, Nakayama R, Yamamoto K, Kumagai H (1997) G-protein coupled receptor from yeast Saccharomyces cerevisiae. Biochem Biophys Res Commun 240(2):287–292
Acknowledgements
This work was supported by the Hungarian National Research, Development and Innovation Office (OTKA K106172), and the European Union, co-financed by the European Social Fund (SROP-4.2.2.B-15/1/KONV-2015-0001). We thank Ilona Lakatos for technical assistance and Dr Tamas Emri (Dept. of Biotechnology and Microbiology, University of Debrecen) for providing the Candida albicans genomic DNA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pataki, E., Sipiczki, M. & Miklos, I. Schizosaccharomyces pombe rsv1 Transcription Factor and its Putative Homologues Preserved their Functional Homology and are Evolutionarily Conserved. Curr Microbiol 74, 710–717 (2017). https://doi.org/10.1007/s00284-017-1227-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-017-1227-9