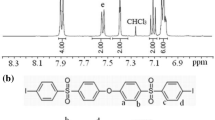
Summary
4,4′-Oxydiphthalic anhydride (1) was reacted with L-methionine (2) in acetic acid and the resulting N,N′-(4,4′-oxydiphthaloyl)-bis-L-methionine diacid (4) was obtained in high yield. The direct polycondensation reaction of this diacid with several aromatic diols such as bisphenol A (5a), phenolphthalein (5b), 1,4-dihydroxyanthraquinone (5c), 4,4′-dihydroxydiphenyl sulfide (5d), 2,6-dihydroxytoluene (5e), 4,4′-dihydroxydiphenyl sulfone (5f) and 2,4′-dihydroxyacetophenone (5g) was carried out in a system of tosyl chloride (TsCl), pyridine (Py) and N,N-dimethylformamide (DMF). The reactions with TsCl were significantly promoted by controlling alcoholysis with diols in the presence of the catalytic amounts of DMF to give a series of optically active poly(ester-imide)s (PEI)s with good yield and moderate inherent viscosity ranging 0.21–0.71 dL/g. The polycondensation reactions were significantly affected by the amounts of DMF, molar concentration of monomers, TsCl and pyridine, aging time, addition time of diols, temperature and the reaction time. All of the above polymers were fully characterized by 1H-NMR, FT-IR, elemental analysis and specific rotation. Some structural characterization and physical properties of these new optically active PEIs are reported.
Similar content being viewed by others
References
Abadie MJM, Sillion B. Polyimides and other high temperature polymers. New York: Elsevier (1991).
Feger C, Khojasteh MM, Molis SE Polyimides, trends in materials and application. New York: Society of Plastic Engineers (1996).
Mallakpour SE, Hajipour AR, Roohipour Fard R (2000) Eur Polym J 2455.
Mallakpour SE, Hajipour AR, Vahabi R (2002) J Appl Polym Sc 84, 35.
Higashi F, Mashimo T (1984) J Polym Sci Part A Polym Chem 24, 1697.
Higashi F, Mashimo T (1985) J Polym Sci Polym Chem Ed 23, 2999.
Higashi F, Akiyama N, Takashi I Koyama T (1984) J Polym Sci Polym Chem Ed 22, 1653.
Higashi F, Ong C H, Okada Y (1999) J Polym Sci Part A Polym Chem 37, 3625.
Okamoto Y, Nakano T (1994) Chem Rev 94, 349.
Okamoto Y, Yashima E (1990) Prog Polym Sci 15, 263.
Yashima E, Maeda K, Okamoto Y (1999) Nature 399, 449.
Mallakpour S, Kowsari E (2005) Polym Bull 53,169.
Mallakpour S, Kowsari E (2005) J Appl Polym Sci 96, 435.
Mallakpour S, Kowsari E (2005) Iran Polym J 14(1), 81.
Mallakpour S, Kowsari E (2004) J Appl Polym Sci 91, 2991.
Mallakpour S, Zamanlou MR (2004) J Appl Polym Sci 91, 3281.
Mallakpour S, Kowsari E (2005) Iranian Polym J 14, 81.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mallakpour, S., Kowsari, E. Synthesis of Organosoluble and Optically Active Poly(ester-imide)s by Direct Polycondensation with Tosyl Chloride in Pyridine and Dimethylformamide. Polym. Bull. 55, 51–59 (2005). https://doi.org/10.1007/s00289-005-0416-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-005-0416-z