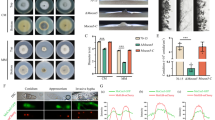
Abstract
Ubiquitination is a key regulatory mechanism that affects numerous important biological processes, including cellular differentiation and pathogenesis in eukaryotic cells. Attachment of proteins to ubiquitin is reversed by specialized proteases, deubiquitinating enzymes (DUBs), which are essential for precursor processing, maintaining ubiquitin homeostasis and promoting protein degradation by recycling ubiquitins. Here, we report the identification of a novel non-pathogenic T-DNA-tagged mutant T612 of Magnaporthe oryzae with a single insertion in the second exon of MoUBP4, which encodes a putative ubiquitin carboxyl-terminal hydrolase. Targeted gene deletion mutants of MoUBP4 are significantly reduced in mycelial growth, conidiation, and increased in tolerance to SDS and CR (Congo red) cell-wall damage. The ΔMoubp4 mutants are blocked in penetration and invasive growth, which results in the loss of pathogenicity. Many conidia produced by the ΔMoubp4 mutants are unable to form appressoria and mobilization and degradation of glycogen and lipid droplets are significantly delayed. Moreover, immunohybridization analysis revealed that total protein ubiquitination levels of the null mutants were significantly increased, indicating that MoUbp4 functions as a deubiquitination enzyme. Taken together, we conclude that MoUbp4 is required for deubiquitination, infection-related morphogenesis and pathogenicity in M. oryzae.









Similar content being viewed by others
References
Amerik AY, Hochstrasser M (2004) Mechanism and function of deubiquitinating enzymes. Biochim Biophys Acta 1695:189–207
Amerik AY, Li SJ, Hochstrasser M (2000a) Analysis of the deubiquitinating enzymes of the yeast Saccharomyces cerevisiae. Biol Chem 381:981–992
Amerik AY, Nowak J, Swaminathan S, Hochstrasser M (2000b) The Doa4 deubiquitinating enzyme is functionally linked to the vacuolar protein-sorting and endocytic pathways. Mol Biol Cell 11:3365–3380
Bryant NJ, Stevens TH (1998) Vacuole biogenesis in Saccharomyces cerevisiae: protein transport pathways to the yeast vacuole. Microbiol Mol Biol Rev 62:230–247
Carroll AM, Sweigard JA, Valent B (1994) Improved vectors for selecting resistance to hygromycin. Fungal Genet Newsl 41:22
Chen Y, Piper PW (1995) Consequences of the overexpression of ubiquitin in yeast: elevated tolerances of osmostress, ethanol and canavanine, yet reduced tolerances of cadmium, arsenite and paromomycin. Biochim Biophys Acta 1268:59–64
Dean RA, Talbot NJ, Ebbole DJ, Farman ML, Mitchell TK, Orbach MJ, Thon M, Kulkarni R, Xu JR, Pan H, Read ND, Lee YH, Carbone I, Brown D, Oh YY, Donofrio N, Jeong JS, Soanes DM, Djonovic S, Kolomiets E, Rehmeyer C, Li W, Harding M, Kim S, Lebrun MH, Bohnert H, Coughlan S, Butler J, Calvo S, Ma LJ, Nicol R, Purcell S, Nusbaum C, Galagan JE, Birren BW (2005) The genome sequence of the rice blast fungus Magnaporthe grisea. Nature 434:980–986
Du Y, Zhang HF, Hong L, Wang JM, Zheng XB, Zhang ZG (2013) Acetolactate synthases MoIlv2 and MoIlv6 are required for infection-related morphogenesis in Magnaporthe oryzae. Mol Plant Pathol 14:870–884
Du Y, Hong L, Tang W, Li LW, Wang XL, Ma HY, Wang ZY, Zhang HF, Zheng XB, Zhang ZG (2014) Threonine deaminase MoIlv1 is important for conidiogenesis and pathogenesis in the rice blast fungus Magnaporthe oryzae. Fungal Genet Biol 73:53–60
Dupré S, Haquenauer-Tsapis R (2001) Deubiquitination step in the endocytic pathway of yeast plasma membrane proteins: crucial role of Doa4p ubiquitin isopeptidase. Mol Cell Biol 21:4482–4494
Ebbole DJ (2007) Magnaporthe as a model for understanding host-pathogen interactions. Annu Rev Phytopathol 45:437–456
Eletr ZM, Wilkinson KD (2014) Regulation of proteolysis by human deubiquitinating enzymes. Biochim Biophys Acta 1843:114–128
Fang W, Price MS, Toffaletti DL, Tenor J, Betancourt-Quiroz M, Price JL, Pan WH, Liao WQ, Perfect JR (2012) Pleiotropic effects of deubiquitinating enzyme Ubp5 on growth and pathogenesis of Cryptococcus neoformans. PLoS One 7:e38326
Finley D, Ozkaynak E, Varshavsky A (1987) The yeast polyubiquitin gene is essential for resistance to high temperatures, starvation, and other stresses. Cell 48:1035–1046
Finley D, Ulrich HD, Sommer T, Kaiser P (2012) The ubiquitin-proteasome system of Saccharomyces cerevisiae. Genetics 192:319–360
Hershko A, Ciechanover A (1998) The ubiquitin system. Annu Rev Biochem 67:425–479
Howard RJ, Valent B (1996) Breaking and entering host penetration by the fungal rice blast pathogen Magnaporthe grisea. Annu Rev Microbiol 50:491–512
Jeon J, Goh J, Yoo S, Chi MH, Choi J, Rho HS, Park J, Han SS, Kim BR, Park SY, Kim S, Lee YH (2008) A putative MAP kinase kinase kinase, MCK1, is required for cell wall integrity and pathogenicity of the rice blast fungus, Magnaporthe oryzae. Mol Plant-Microbe Interact 21:525–534
Katzmann DJ, Babst M, Emr SD (2001) Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 106:145–155
Kim S, Park SY, Kim KS, Rho HS, Chi MH, Choi J, Park J, Kong S, Park J, Goh J, Lee YH (2009) Homeobox transcription factors are required for conidiation and appressorium development in the rice blast fungus Magnaporthe oryzae. PLoS Genet 5:e1000757
Kimura Y, Yashiroda H, Kudo T, Koitabashi S, Murata S, Kakizuka A, Tanaka K (2009) An inhibitor of a deubiquitinating enzyme regulates ubiquitin homeostasis. Cell 137:549–559
Latgé JP (2007) The cell wall: a carbohydrate armour for the fungal cell. Mol Microbiol 66:279–290
Leung H, Borromeo ES, Bernardo MA, Notteghem JL (1988) Genetic analysis of virulence in the rice blast fungus Magnaporthe grisea. Phytopathology 78:1227–1233
Li Y, Liang S, Yan X, Wang H, Li DB, Soanes DM, Talbot NJ, Wang ZH, Wang ZY (2010) Characterization of MoLDB1 required for vegetative growth, infection-related morphogenesis, and pathogenicity in the rice blast fungus Magnaporthe oryzae. Mol Plant-Microbe Interact 23:1260–1274
Liu YG, Chen Y (2007) High-efficiency thermal asymmetric interlaced PCR for amplification of unknown flanking sequences. Biotechniques 43:649–656
Liu OW, Chun CD, Chow ED, Chen C, Madhani HD, Noble SM (2008a) Systematic genetic analysis of virulence in the human fungal pathogen Cryptococcus neoformans. Cell 135:174–188
Liu Y, Wang F, Zhang H, He H, Ma L, Deng XW (2008b) Functional characterization of the Arabidopsis ubiquitin-specific protease gene family reveals specific role and redundancy of individual members in development. Plant J 55:844–856
Liu WD, Xie SY, Zhao XH, Chen X, Zheng WH, Lu GD, Xu JR, Wang ZH (2010) A homeobox gene is essential for conidiogenesis of the rice blast fungus Magnaporthe oryzae. Mol Plant-Microbe Interact 23:366–375
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Luhtala N, Odorizzi G (2004) Bro1 coordinates deubiquitination in the multivesicular body pathway by recruiting Doa4 to endosomes. J Cell Biol 166:717–729
McCafferty HR, Talbot NJ (1998) Identification of three ubiquitin genes of the rice blast fungus Magnaporthe grisea, one of which is highly expressed during initial stages of plant colonization. Curr Genet 33:352–361
Mukhopadhyay D, Riezman H (2007) Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science 315:201–205
Nikko E, André B (2007) Evidence for a direct role of the Doa4 deubiquitinating enzyme in protein sorting into the MVB pathway. Traffic 8:566–581
Nishimura M, Fukada J, Moriwaki A, Fujikawa T, Ohashi M, Hibi T, Hayashi N (2009) Mstu1, an APSES transcription factor, is required for appressorium-mediated infection in Magnaporthe grisea. Biosci Biotechnol Biochem 73:1779–1786
Odenbach D, Breth B, Thines E, Weber RW, Anke H, Foster AJ (2007) The transcription factor Con7p is a central regulator of infection-related morphogenesis in the rice blast fungus Magnaporthe grisea. Mol Microbiol 64:293–307
Odorizzi G, Katzmann DJ, Babst M, Audhya A, Emr SD (2003) Bro1 is an endosome-associated protein that functions in the MVB pathway in Saccharomyces cerevisiae. J Cell Sci 116:1893–1903
Oh Y, Franck WL, Han SO, Shows A, Gokce E, Muddiman DC, Dean RA (2012) Polyubiquitin is required for growth, development and pathogenicity in the rice blast fungus Magnaporthe oryzae. PLoS One 7:e42868
Ozkaynak E, Finley D, Solomon MJ, Varshavsky A (1987) The yeast ubiquitin genes: a family of natural gene fusions. EMBO J 6:1429–1439
Pan YM, Pan R, Tan LY, Zhang ZG, Guo M (2019) Pleiotropic roles of O-mannosyltransferase MoPmt4 in development and pathogenicity of Magnaporthe oryzae. Curr Genet 65:223–239
Park G, Xue C, Zheng L, Lam S, Xu JR (2002) MST12 regulates infectious growth but not appressorium formation in the rice blast fungus Magnaporthe grisea. Mol Plant-Microbe Interact 15:183–192
Park G, Bruno KS, Staiger CJ, Talbot NJ, Xu JR (2004) Independent genetic mechanisms mediate turgor generation and penetration peg formation during plant infection in the rice blast fungus. Mol Microbiol 53:1695–1707
Pashkova N, Gakhar L, Winistorfer SC, Sunshine AB, Rich M, Dunham MJ, Yu L, Piper RC (2013) The yeast Alix homolog Bro1 functions as a ubiquitin receptor for protein sorting into multivesicular endosomes. Dev Cell 25:520–533
Prakash C, Manjrekar J, Chattoo BB (2016) Skp1, a component of E3 ubiquitin ligase, is necessary for growth, sporulation, development and pathogenicity in rice blast fungus (Magnaporthe oryzae). Mol Plant Pathol 17:903–919
Ren WC, Sang CW, Shi DY, Song XS, Zhou MG, Chen CJ (2018) Ubiquitin-like activating enzymes BcAtg3 and BcAtg7 participate in development and pathogenesis of Botrytis cinerea. Curr Genet 64:919–930
Ryu KY, Baker RT, Kopito RR (2006) Ubiquitin-specific protease 2 as a tool for quantification of total ubiquitin levels in biological specimens. Anal Biochem 353:153–155
Sesma A, Osbourn AE (2004) The rice leaf blast pathogen undergoes developmental processes typical of root-infecting fungi. Nature 431:582–586
Shi ZX, Christian D, Leung H (1998) Interactions between spore morphogenetic mutations affect cell types, sporulation, and pathogenesis in Magnaporthe grisea. Mol Plant-Microbe Interact 11:199–207
Shi HB, Chen GQ, Chen YP, Dong B, Lu JP, Liu XH, Lin FC (2016) MoRad6-mediated ubiquitination pathways are essential for development and pathogenicity in Magnaporthe oryzae. Environ Microbiol 18:4170–4187
Springael JY, Nikko E, André B, Marini AM (2002) Yeast Npi3/Bro1 is involved in ubiquitin-dependent control of permease trafficking. FEBS Lett 517:103–109
Swaminathan S, Amerik AY, Hochstrasser M (1999) The Doa4 deubiquitinating enzyme is required for ubiquitin homeostasis in yeast. Mol Biol Cell 10:2583–2594
Talbot NJ (1995) Having a blast: exploring the pathogenicity of Magnaporthe grisea. Trends Microbiol 3:9–16
Talbot NJ (2003) On the trail of a cereal killer: exploring the biology of Magnaporthe grisea. Annu Rev Microbiol 57:177–202
Talbot NJ, Ebbole DJ, Hamer JE (1993) Identification and characterization of MPG1, a gene involved in pathogenicity from the rice blast fungus Magnaporthe grisea. Plant Cell 5:1575–1590
Thines E, Weber RW, Talbot NJ (2000) MAP kinase and protein kinase A-dependent mobilization of triacylglycerol and glycogen during appressorium turgor generation by Magnaporthe grisea. Plant Cell 12:1703–1718
Wang XL, Xu X, Liang YM, Wang YL, Tian CM (2018a) A Cdc42 homolog in Colletotrichum gloeosporioides regulates morphological development and is required for ROS-mediated plant infection. Curr Genet 64:1153–1169
Wang Z, Zhang H, Liu C, Xing J, Chen XL (2018b) A deubiquitinating enzyme Ubp14 is required for development, stress response, nutrient utilization, and pathogenesis of Magnaporthe oryzae. Front Microbiol 9:769
Wemmer M, Azmi I, West M, Davies B, Katzmann D, Odorizzi G (2011) Bro1 binding to Snf7 regulates ESCRT-III membrane scission activity in yeast. J Cell Biol 192:295–306
Wilson RA, Talbot NJ (2009) Under pressure: investigating the biology of plant infection by Magnaporthe oryzae. Nat Rev Microbiol 7:185–195
Wolters N, Amerik AY (2016) Inactivation of the VID27 gene prevents suppression of the doa4 degradation defect in doa4Δdid3Δ double mutant. Biochem Biophys Res Commun 482:1341–1345
Xu JR, Hamer JE (1996) MAP kinase and cAMP signaling regulate infection structure formation and pathogenic growth in the rice blast fungus Magnaporthe grisea. Genes Dev 10:2696–2706
Yang J, Zhao XY, Sun J, Kang ZS, Ding SL, Xu JR, Peng YL (2010) A novel protein Com1 is required for normal conidium morphology and full virulence in Magnaporthe oryzae. Mol Plant-Microbe Interact 23:112–123
Yu JH, Hamari Z, Han KH, Seo JA, Reyes-Domínguez Y, Scazzocchio C (2004) Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet Biol 41:973–981
Yue XF, Que YW, Xu L, Deng SZ, Peng YL, Talbot NJ, Wang ZY (2016) ZNF1 encodes a putative C2H2 zinc-finger protein essential for appressorium differentiation by the rice blast fungus Magnaporthe oryzae. Mol Plant-Microbe Interact 29:22–35
Zhang N, Luo J, Rossman AY, Aoki T, Chuma I, Crous PW, Dean R, de Vries RP, Donofrio N, Hyde KD, Lebrun MH, Talbot NJ, Tharreau D, Tosa Y, Valent B, Wang ZH, Xu JR (2016) Generic names in Magnaporthales. IMA Fungus 7:155–159
Zhao TT, Tian HT, Xia YX, Jin K (2019) MaPmt4, a protein O-mannosyltransferase, contributes to cell wall integrity, stress tolerance and virulence in Metarhizium acridum. Curr Genet 65:1025–1040
Zheng WH, Lin YH, Fang WQ, Zhao X, Lou Y, Wang GH, Zheng HW, Liang QF, Abubabar YS, Olsson S, Zhou J, Wang ZH (2018) The endosomal recycling of FgSnc1 by FgSnx41-FgSnx4 heterodimer is essential for polarized growth and pathogenicity in Fusarium graminearum. New Phytol 219:654–671
Zhou ZZ, Li GH, Lin CH, He CZ (2009) Conidiophore stalk-less1 encodes a putative Zinc-Finger protein involved in the early stage of conidiation and mycelial infection in Magnaporthe oryzae. Mol Plant-Microbe Interact 22:402–410
Zhou H, Zhao J, Cai J, Patil SB (2017) Ubiquitin-specific proteases function in plant development and stress responses. Plant Mol Biol 94:565–576
Acknowledgements
This work was supported by the Natural Science Foundation of China (Grant Nos. 31570135 and 31770153) to ZW. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: YQ, ZX, and ZW. Performed the experiments: YQ, ZX, CW, WL, XY, LX, ST, HD, and ZW. Analyzed the data: YQ, ZX, and ZW. Wrote the paper: YQ, ZX, and ZW.
Corresponding author
Additional information
Communicated by M. Kupiec.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
294_2019_1049_MOESM1_ESM.docx
Supplementary material 1 Figure S1. Phylogenetic analysis of MoUbp4 with the homologues from other fungal species. Phylogenetic tree of MoUbp4 and its homologs from other fungal species was constructed by the neighbor-joining method using the MEGA version 5.0 program. Numbers at the nodes in the rooted tree represent bootstrapping value on 1000 replications. GenBank accession numbers are presented after fungal species names. The percentage in bracket indicates the sequence identity between MoUbp4 with its homologues in other species. The bar indicates 0.2 distance units (DOCX 15 kb)
294_2019_1049_MOESM2_ESM.tif
Supplementary material 2 Figure S2. Construction of the MoUBP4 gene deletion vector and generation of the targeted gene deletion mutants. (A) Construction of the vector pUBP4-KO and targeted gene replacement of MoUBP4. P, PstI. (B) PCR analysis of the wild-type strain (Guy11), ΔMoubp4 mutants (-1, -17, -24) and an ecotopic transformant (ECT-5) by primer pairs of UBP4yqdx-F/UBP4yqdx-R (top panel) and UBP4yq-F/UBP4yq-F (bottom). (C) Southern blot analysis. Genomic DNAs of different strains were digested with PstI and hybridized with a 998 bp probe amplified with the primers Probe-F and Probe-R (TIFF 27857 kb)
294_2019_1049_MOESM3_ESM.tif
Supplementary material 3 Figure S3. MoUBP4 is not required for the sexual reproduction in M. oryzae. (A) Sexual reproduction assay. The wild-type Guy11 (MAT1-2) and ΔMoubp4 mutants were crossed with a standard tester strain, TH3 (MAT1-1) to allow perithecium production. By 30 days following inoculation, numerous perithecia and ascospores at the junctions of both the crosses of Guy11 × TH3 and ΔMoubp4 × TH3, indicating that MoUBP4 is not required for development of fruiting bodies by M. oryzae. Arrows indicate perithecia. (B) Microscopic observation of ascospore development. Scale bar = 10 μm (TIFF 30307 kb)
294_2019_1049_MOESM4_ESM.tif
Supplementary material 4 Figure S4. Deletion of MoUBP4 does not result in alteration of appressorium turgor pressure in M. oryzae. Statistical analysis of collapsed appressoria (24 h) of the ΔMoubp4 mutants after incubation for 15 min in 1, 2 or 3 M glycerol solutions. Error bars represent standard deviation (TIFF 26663 kb)
294_2019_1049_MOESM5_ESM.tif
Supplementary material 5 Figure S5. MoUbp4 interacts with MoBro1 in M. oryzae. Physical interaction among MoUbp4, MoBro1 and MoSnf7 was detected by Y2H assays. The yeast transformants were assayed for growth on SD/-Ade/-His/-Trp/-Leu medium. Positive control, pGBKT7-53/pGADT7. Negative control, pGBKT7-lam/pGADT7 (TIFF 28334 kb)
Rights and permissions
About this article
Cite this article
Que, Y., Xu, Z., Wang, C. et al. The putative deubiquitinating enzyme MoUbp4 is required for infection-related morphogenesis and pathogenicity in the rice blast fungus Magnaporthe oryzae. Curr Genet 66, 561–576 (2020). https://doi.org/10.1007/s00294-019-01049-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-019-01049-8