Abstract
Objective
To enhance the positive predictive value (PPV) of chest digital tomosynthesis (DTS) in the lung cancer detection with the analysis of radiomics features.
Method
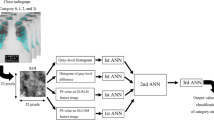
The investigation was carried out within the SOS clinical trial (NCT03645018) for lung cancer screening with DTS. Lung nodules were identified by visual analysis and then classified using the diameter and the radiological aspect of the nodule following lung-RADS. Haralick texture features were extracted from the segmented nodules. Both semantic variables and radiomics features were used to build a predictive model using logistic regression on a subset of variables selected with backward feature selection and using two machine learning: a Random Forest and a neural network with the whole subset of variables. The methods were applied to a train set and validated on a test set where diagnostic accuracy metrics were calculated.
Results
Binary visual analysis had a good sensitivity (0.95) but a low PPV (0.14). Lung-RADS classification increased the PPV (0.19) but with an unacceptable low sensitivity (0.65). Logistic regression showed a mildly increased PPV (0.29) but a lower sensitivity (0.20). Random Forest demonstrated a moderate PPV (0.40) but with a low sensitivity (0.30). Neural network demonstrated to be the best predictor with a high PPV (0.95) and a high sensitivity (0.90).
Conclusions
The neural network demonstrated the best PPV. The use of visual analysis along with neural network could help radiologists to reduce the number of false positive in DTS.
Key Points
• We investigated several approaches to enhance the positive predictive value of chest digital tomosynthesis in the lung cancer detection.
• Neural network demonstrated to be the best predictor with a nearly perfect PPV.
• Neural network could help radiologists to reduce the number of false positive in DTS.



Similar content being viewed by others
Abbreviations
- AIC:
-
Akaike Information Criteria
- AUC:
-
Area under the curve
- CAD:
-
Computer-aided detection
- CT:
-
Computed tomography
- DTS:
-
Digital tomosynthesis
- GGO:
-
Ground-glass opacity
- GLCM:
-
Gray-level co-occurrence matrix
- GLRLM:
-
Gray-level run-length matrix
- GLSZM:
-
Gray-level size zone matrix
- LR:
-
Logistic regression
- NGLDM:
-
Neighboring gray-level dependence matrix
- NLST:
-
National Lung Screening Trial
- NNET:
-
Neural network
- PPV:
-
Positive predictive value
- RF:
-
Random Forest
References
Fitzmaurice C, Allen C, Barber RM et al (2017) Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease study. JAMA Oncol 3:524–548. https://doi.org/10.1001/jamaoncol.2016.5688
Aberle DR, Adams AM, Berg CD et al (2011) Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 365:395–409
Church TR, Black WC, Aberle DR et al (2013) Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 368:1980–1991. https://doi.org/10.1056/NEJMoa1209120
Bach PB, Mirkin JN, Oliver TK et al (2012) Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 307:2418–2429. https://doi.org/10.1001/jama.2012.5521
Terzi A, Bertolaccini L, Viti A et al (2013) Lung cancer detection with digital chest tomosynthesis: baseline results from the observational study SOS. J Thorac Oncol 8:685–692. https://doi.org/10.1097/JTO.0b013e318292bdef
Grosso M, Priotto R, Ghirardo D et al (2017) Comparison of digital tomosynthesis and computed tomography for lung nodule detection in SOS screening program. Radiol Med 122:568–574. https://doi.org/10.1007/s11547-017-0765-3
McKee BJ, McKee AB, French R et al (2012) “Lung-RADS” a proposed standardized reporting and data system for CT lung cancer screening. J Thorac Oncol 4:S277–S278
MacMahon H, Austin JHM, Gamsu G et al (2005) Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 237:395–400
Hatt M, Tixier F, Pierce L, Kinahan PE, Le Rest CC, Visvikis D (2017) Characterization of PET/CT images using texture analysis: the past, the present … any future ? Eur J Nucl Med Mol Imaging 44:151–165. https://doi.org/10.1007/s00259-016-3427-0
Haralick RM, Dinstein I, Shanmugam K (1973) Textural features for image classification. IEEE Trans Syst Man Cybern. https://doi.org/10.1109/TSMC.1973.4309314
Menardi G, Torelli N (2014) Training and assessing classification rules with imbalanced data. Data Min Knowl Disc. https://doi.org/10.1007/s10618-012-0295-5
Kendall MG (1945) The treatment of ties in ranking problems. Biometrika 33:239–251. https://doi.org/10.1093/biomet/33.3.239
Moons KGM, Altman DG, Reitsma JB et al (2015) Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 162:W1–W73. https://doi.org/10.7326/M14-0698
Kim JH, Lee KH, Kim KT et al (2016) Comparison of digital tomosynthesis and chest radiography for the detection of pulmonary nodules: systematic review and meta-analysis. Br J Radiol 89. https://doi.org/10.1259/bjr.20160421
Pinsky PF, Gierada DS, Black W et al (2015) Performance of lung-RADS in the national lung screening trial: a retrospective assessment. Ann Intern Med 162:485–491. https://doi.org/10.7326/M14-2086
Al Mohammad B, Brennan PC, Mello-Thoms C (2017) A review of lung cancer screening and the role of computer-aided detection. Clin Radiol 72:433–442. https://doi.org/10.1016/j.crad.2017.01.002
Wang J, Dobbins JT 3rd, Li Q (2012) Automated lung segmentation in digital chest tomosynthesis. Med Phys 39:732–741. https://doi.org/10.1118/1.3671939
Hadházi D, Varga R, Horváth A, Czétényi B, Horváth G (2015) Digital chest tomosynthesis: the main steps to a computer assisted lung diagnostic system. In: 2015 IEEE International Symposium on Medical Measurements and Applications, MeMeA 2015 - Proceedings. pp 40–45
Horváth Á, Wolf P, Nagy J et al (2016) Overview of a digital tomosynthesis development: new approaches for low-dose chest imaging. Radiat Prot Dosimetry 169:171–176. https://doi.org/10.1093/rpd/ncv469
Balagurunathan Y, Schabath MB, Wang H, Liu Y, Gillies RJ (2019) Quantitative imaging features improve discrimination of malignancy in pulmonary nodules. Sci Rep 9:1–14. https://doi.org/10.1038/s41598-019-44562-z
Lu H, Mu W, Balagurunathan Y et al (2019) Multi-window CT based Radiomic signatures in differentiating indolent versus aggressive lung cancers in the National Lung Screening Trial: a retrospective study. Cancer Imaging:19. https://doi.org/10.1186/s40644-019-0232-6
Wu W, Pierce LA, Zhang Y et al (2019) Comparison of prediction models with radiological semantic features and radiomics in lung cancer diagnosis of the pulmonary nodules: a case-control study. Eur Radiol. https://doi.org/10.1007/s00330-019-06213-9
Acknowledgments
A special thanks to the technologists Kawtar Nourani and Denise Guerra for their precious collaboration.
SOS Study team: Alberto Biggi (SC Medicina Nucleare), Andrea Campione, Mirella Fortunato (SC Anatomia Patologica), Adriano De Maggi, Stéphane Chauvie (SC Fisica Sanitaria), Ida Colantonio (SC Oncologia), Maurizio Grosso (SC Radiologia), Giulio Melloni, Federico Mazza, Alessia Stanzi (SC Chirurgia Toracica), Paolo Noceti (SC Pneumologia), Paolo Pellegrino (Direzione Sanitaria), Elvio Russi (SC Radioterapia)
Funding
This study has received funding from “Cassa di Risparmio di Cuneo” Foundation. Santa Croce e Carle, the hospital where the study was performed, provided logistic support, telephone lines, software, computer assistance, and an office free of charge.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Maurizio Grosso.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in the results of the SOS study which have already been published as specified in the references. This study focuses on the application of AI to the data obtained during the trial.
Methodology
• Prospective
• Diagnostic or prognostic study
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chauvie, S., De Maggi, A., Baralis, I. et al. Artificial intelligence and radiomics enhance the positive predictive value of digital chest tomosynthesis for lung cancer detection within SOS clinical trial. Eur Radiol 30, 4134–4140 (2020). https://doi.org/10.1007/s00330-020-06783-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-06783-z